From the journals: MCP
Lysosomal proteins differ across cell lines. Reducing background in protein identification. Stress-related differences in protein sugars. Read about papers on these topics recently published in the journal Molecular & Cellular Proteomics.
Stress-related differences in protein sugars
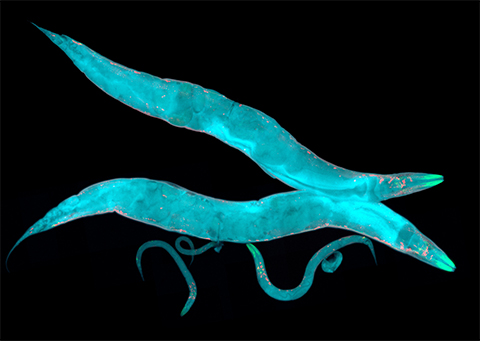
Protein glycosylation is the enzymatic addition of sugars, or glycans, to a target protein after translation that influences how the protein folds and functions. The extent of protein glycosylation can change in an organism during different developmental stages. Iain Wilson from the University of Natural Resources and Life Sciences in Vienna, Austria, and an international team of researchers discovered that the way the nematode Caenorhabditis elegans is cultivated also influences the level of glycosylation in its cells. In a recent paper in the journal Molecular & Cellular Proteomics, the researchers described their findings on the range of N-glycans, linked to the asparagine amino acids of proteins, in C. elegans from embryo to larva.
C. elegans contains only about 1,000 body cells and has a complex glycosylation profile, or glycome, including over 200 different N-glycan structures. Previous studies have investigated the level of RNA transcripts of glycosylation enzymes. While the set of glycan structures found in C. elegans does not correlate with the number of enzyme transcripts, researchers have found evidence that glycosylation-relevant genes are expressed in a coordinated way.
To profile glycomes, Wilson’s team cut the glycans from collected proteins using enzymes called peptide-N-glycosidases, or PNGases. The researchers discovered no difference in the embryonal glycome of wild-type C. elegans and two mutants with developmental defects. However, they found a clear difference in the glycome between liquid- and plate-grown worms in the fourth larval stage, known as L4. Liquid-grown L4 larvae have about three times more structures with a galactose sugar in an alpha position linked to mannose, and/or methylation of other sugar units, than plate-grown larvae. The researchers hypothesize that this shift is stress related, having to do with the different conditions under which the C. elegans is grown; being shaken in liquid is unlike their natural soil habitat!
Lysosomal proteins differ across cell lines
Lysosomes are the main organelles responsible for degrading and recycling compounds in mammalian cells. Lysosomal malfunction can result in rare diseases and has been shown to impact more common diseases, including cancer. However, all lysosomes are not the same between cell types. In a new study in the journal Molecular & Cellular Proteomics, Fatema Akter, Sara Bonini and Srigayatri Ponnaiyan from the University of Bonn, in collaboration with researchers from the Bangladesh Agricultural University and the University of Kiel, identified a distinct population of lysosomal proteins over six cell lines and discovered many potentially novel lysosomal proteins.
Using nanoparticles for protein binding, the researchers identified lysosomal proteins in the cell lines HEK293, HeLa, HuH-7, SH-SY5Y, MEF and NIH3T3 with mass spectrometry, reverse transcription polymerase chain reaction and immunostaining techniques. Most of the identified proteins were detected across all the cell lines, forming a core proteome that is highly conserved among the lysosomes of different lines. SH-SY5Y cells were the most similar to other cell lines, and NIH3T3 cells were least similar. The researchers analyzed protein abundance across functions as well, finding patterns in the abundance of transporter proteins, the mammalian target of rapamycin complex and hydrolysis of glycosidic bonds.
Reducing background in protein identification
When a cell is exposed to stimuli or stress, the pattern of messenger RNA expression and protein synthesis changes. Newly synthesized, or nascent, proteins can provide important information on what biochemical processes are occurring when the cell responds. Profiling these proteins in the presence of preexisting cellular proteins requires specific labeling and purification protocols. Nancy J. Phillips and the team from the University of California, San Francisco, optimized a method for nascent protein detection by incorporating a cleavable linker molecule, as described in a recent paper in the journal Molecular & Cellular Proteomics.
To selectively label nascent proteins, the researchers introduced the antibiotic analog O-propargyl-puromycin, or OPP, to the cell. It entered the A-site of the ribosome and was incorporated randomly at the C terminus of the polypeptide chain. This resulted in truncated, OPP-labeled nascent proteins. The researchers then used click chemistry to attach a biotin molecule with a cleavable linker. This biotin molecule could be captured on streptavidin beads. The beads could then be washed and the nascent proteins selectively released by chemical cleavage, leaving behind nonspecific binders. Purified proteins were then characterized by liquid chromatography–mass spectrometry. This method greatly reduced background protein levels and enabled analysis of nascent proteins at low concentrations.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

