For June and July, it’s atomic Nos. 6 and 7
This series of articles on chemical elements important for life marks the International Year of the Periodic Table in 2019. To date, we have introduced hydrogen, iron, sodium, potassium, chlorine, copper, calcium and phosphorus. This month, we continue the series with carbon and nitrogen.
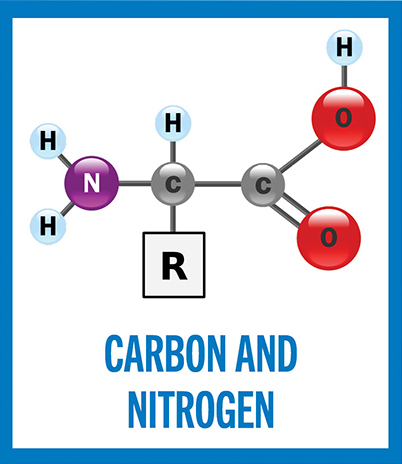 General structure of an amino acid (with the exception of proline). The alpha carbon in the center is bonded to four groups: an amino group (NH2), a carboxyl group (COOH), a hydrogen atom (H) and a variable side chain (R group). The R group determines the overall structure, size, electric charge and polarity of the amino acid (in glycine, R is another hydrogen atom).
General structure of an amino acid (with the exception of proline). The alpha carbon in the center is bonded to four groups: an amino group (NH2), a carboxyl group (COOH), a hydrogen atom (H) and a variable side chain (R group). The R group determines the overall structure, size, electric charge and polarity of the amino acid (in glycine, R is another hydrogen atom).
Carbon, with symbol C and atomic No. 6, and nitrogen, with symbol N and atomic No. 7, are reactive nonmetals that generally share electrons with other atoms by forming several covalent bonds. A carbon atom has four electrons and four vacancies in its outermost shell and can combine with other atoms via four covalent bonds. Nitrogen has five valence electrons in its outermost orbitals, three of which are high-energy unpaired electrons that each can form a covalent bond. Nitrogen typically connects with other atoms through these bonds.
Carbon is the fourth most abundant element in the observable universe and the 15th most common in the Earth’s crust. Carbon is produced in the core of stars when two helium nuclei collide to form highly unstable beryllium, which fuses with another helium particle to produce a stable carbon nucleus. When massive stars explode as supernovae, carbon disperses into space. It accumulates in the Earth’s atmosphere where it combines with oxygen as carbon dioxide, or CO2. Carbon is dissolved in all of Earth’s water bodies and is a common constituent of large carbonate rocks — such as limestone and marble — and coal.
Nitrogen is produced in supermassive stars when carbon reacts with hydrogen to produce nitrogen, oxygen and helium in a cycle that generates light and heat. Nitrogen is the seventh most abundant element in the Milky Way and the solar system, and it represents about 78% of the Earth’s atmosphere in the form of dinitrogen gas, or N2. Nitrogen makes up about 0.002% of the Earth’s crust, where it is trapped from the atmosphere during mineral formation, and it dissolves in the oceans via precipitation or sediment runoff.
Both carbon and nitrogen are exchanged continuously in the environment. In the carbon cycle, atmospheric carbon dioxide is converted to organic carbon via photosynthesis. Organisms cycle carbon back into the atmosphere or soil by respiration, excretion and decomposition. In addition, carbon trapped in fossil fuel reservoirs during older geological events is released continuously into the environment by erosion, volcanic emission and human activity, contributing to the present cycle.
Unlike carbon, atmospheric nitrogen cannot be used directly by plants. Instead, nitrogen gas is combined with oxygen or hydrogen by soil bacteria and converted into nitrates and ammonia, respectively. Plants — and subsequently animals — use fixed nitrogen to build biological molecules such as proteins and nucleic acids. Organic nitrogen is returned to the soil by excretion and decomposition and cycled back into ammonia by bacteria and fungi.
The chemistry of life is organized around carbon. Carbon atoms can react with each other to form very stable carbon–carbon bonds. Multiple carbon atoms linked together can exist as linear chains, branched trees and cyclic rings. These structures, called carbon skeletons or backbones, combine with various chemical groups to make the biomolecules and organic compounds found in living cells.
In addition to carbon–carbon interactions, carbon forms single bonds with hydrogen atoms and both single and double bonds with oxygen and nitrogen. Carbon skeletons bonded only to hydrogen are called hydrocarbons, and they include the tails of fatty acids, methane and methyl groups. Other biomolecules contain carbon atoms bonded to oxygen in several chemical arrangements, including hydroxyl groups in alcohols such as glycerol, carbonyl groups in aldehydes and ketones represented in monosaccharides, and carboxyl groups in carboxylic acids like in amino acids.
Nitrogen combines with carbon, forming ring configurations such as purines and pyrimidines that constitute nucleic acids. Biomolecules that contain carbon and nitrogen also include amines and amides. Amines contain the amino group R-NH2, which ionizes to R-NH3+ in aqueous solutions due to its reaction with an H+ from water. Amines occur in amino acids and some neurotransmitters. Amides are formed by the combination of an acid and an amine, such as during formation of peptide bonds that join amino acids in proteins. Enzymatic deamination of the amino acid arginine produces nitric oxide gas that readily diffuses across cellular membranes, acting as a signaling molecule. In animals, nitric oxide regulates smooth muscle contraction and activates macrophages and neutrophils to kill microorganisms.
Carbon and nitrogen occur in all known life. Together with hydrogen and oxygen, they form stable compounds commonly found in biological molecules, where the four elements combined make up between 96% and 99% of any living organism’s mass.
A year of (bio)chemical elements
Read the whole series:
For January, it’s atomic No. 1
For February, it’s iron — atomic No. 26
For March, it’s a renal three-fer: sodium, potassium and chlorine
For April, it’s copper — atomic No. 29
For May, it’s in your bones: calcium and phosphorus
For June and July, it’s atomic Nos. 6 and 7
Breathe deep — for August, it’s oxygen
Manganese seldom travels alone
For October, magnesium helps the leaves stay green
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Building better tools to decipher the lipidome
Chemical engineer–turned–biophysicist Matthew Mitsche uses curiosity, coding and creativity to tackle lipid biology, uncovering PNPLA3’s role in fatty liver disease and advancing mass spectrometry tools for studying complex lipid systems.

Redefining lipid biology from droplets to ferroptosis
James Olzmann will receive the ASBMB Avanti Award in Lipids at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Women’s health cannot leave rare diseases behind
A physician living with lymphangioleiomyomatosis and a basic scientist explain why patient-driven, trial-ready research is essential to turning momentum into meaningful progress.

Life in four dimensions: When biology outpaces the brain
Nobel laureate Eric Betzig will discuss his research on information transfer in biology from proteins to organisms at the 2026 ASBMB Annual Meeting.

