For January, it’s atomic No. 1
Following a proposal initiated by the International Union of Pure and Applied Chemistry and other global scientific organizations, the United Nations has declared 2019 the International Year of the Periodic Table of Chemical Elements, or IYPT2019.
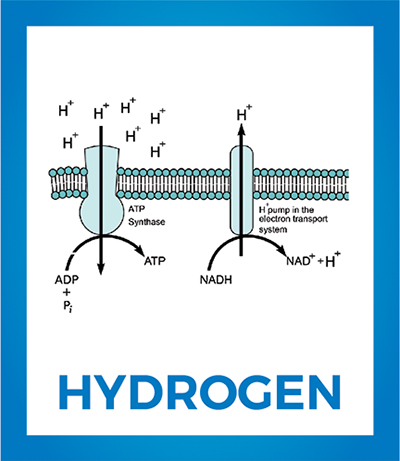 High-energy electrons (from the oxidation of food, for example) passed along the electron-transport chain release energy that is used to pump H+ across the membrane. The resulting electrochemical proton gradient serves as an energy store used to drive adenosine triphosphate synthesis by the adenosine triphosphate synthase. Wikipedia
High-energy electrons (from the oxidation of food, for example) passed along the electron-transport chain release energy that is used to pump H+ across the membrane. The resulting electrochemical proton gradient serves as an energy store used to drive adenosine triphosphate synthesis by the adenosine triphosphate synthase. Wikipedia
The designation commemorates the 150th anniversary of the first publication of Russian chemist Dmitri Mendeleev’s periodic table in 1869. Mendeleev’s table was not the first attempt to arrange the just over 60 chemical elements known at the time, but it was the first version to predict the existence of unidentified elements based on the periodicity of the elements’ physical and chemical properties in relation to their atomic mass.
Today’s periodic table contains at least 118 confirmed elements; of these, only about 30 are essential to living organisms. Bulk elements such as hydrogen, carbon, nitrogen and oxygen are abundant structural components of cells and tissues, whereas trace elements (iron, zinc, copper and magnesium, for example) occur in minute amounts as enzyme cofactors and stabilizing centers for protein complexes.
To celebrate IYPT2019, we are launching a yearlong series that features at least one monthly element with an important role in the molecular life sciences.
Hydrogen
For January, we selected the first element of the periodic table, hydrogen, whose atomic number 1 indicates the presence of a single proton in its nucleus. Hydrogen can occur as a single atom designated as H, as diatomic gas, or H2, in molecules such as water or natural organic compounds (such as carbohydrates, lipids and amino acids) or as negative or positive ions — H- or H+, respectively — in ionic compounds.
Living organisms use hydrogen in oxidation-reduction, or redox, reactions and electrochemical gradients to derive energy for growth and work. Microbes can uptake H2 from the environment and use it as a source of electrons in redox interconversions catalyzed by enzymes called hydrogenases. The transfer of electrons between H2 and acceptor molecules generates H+, and it’s accompanied by substantial energy changes that can be used for cellular metabolism such as synthesis of molecules, cell movement and solute transport.
Cells also use H+ to generate energy from the breakdown of foods such as sugars, fats and amino acids in a process called cellular respiration. In a cascade of metabolic reactions, nutrients like glucose are oxidized and split into smaller molecules, yielding reduced nicotinamide adenine dinucleotide, or NADH, and reduced flavin adenine dinucleotide, or FADH2 as biochemical intermediates.
Under aerobic conditions, a series of proteins that comprise the electron transport chain transfer electrons from NADH and FADH2 to cellular oxygen while pumping H+ across a membrane. This process generates a strong H+ electrochemical gradient with enough force to drive the activity of the adenosine triphosphate synthase, resulting in biochemical energy production as the gradient dissipates.
The potential energy in H+ gradients can be used to generate heat for thermogenesis in the brown fat tissue of hibernating mammals, to power flagellar motors in bacteria, to transport nutrients into cells or to generate low pH inside vacuoles. These examples highlight the ubiquitous role of a single element — hydrogen — in essential-for-life biochemical reactions across multiple kingdoms.
A year of (bio)chemical elements
Read the whole series:
For January, it’s atomic No. 1
For February, it’s iron — atomic No. 26
For March, it’s a renal three-fer: sodium, potassium and chlorine
For April, it’s copper — atomic No. 29
For May, it’s in your bones: calcium and phosphorus
For June and July, it’s atomic Nos. 6 and 7
Breathe deep — for August, it’s oxygen
Manganese seldom travels alone
For October, magnesium helps the leaves stay green
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

