Peering into ocular waste recycling
A recent study in the Journal of Biological Chemistry revealed the key to a protein that commonly causes blindness. The biological process involves a protein that is essential for transporting toxic compounds out of the eye, similar to a garbage recycling service. The challenge is that, like food and the waste it generates, these compounds are essential for the eye to function properly — until they build up and cause blindness.
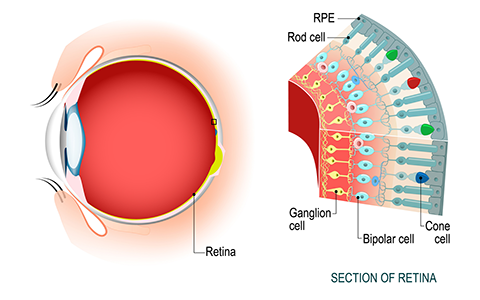
The scientists behind the study research a protein transporter, called ABCA4, that lines the edges of specialized photoreceptor cells in the retina and is normally poised to remove toxic, fatty retinal byproducts called N-Ret-PE. Retinal is a derivative of vitamin A, which is found in foods such as leafy green vegetables.
“Retinal is critical for vision,” said Robert Molday, a professor of biochemistry and molecular biology at the University of British Columbia who oversaw the work. “But, it's also potentially very toxic because it has a very reactive element. So, cells have to be able to balance between using retinal for sustained vision as well as managing its toxicity .”
Mutations in ABCA4 can cause N-Ret-PE buildup, which leads to vision loss in diseases such as Stargardt disease. Stargardt disease is the most common inherited form of macular degeneration and affects approximately 30,000 people nationwide. There is currently no therapy or cure for the disease.
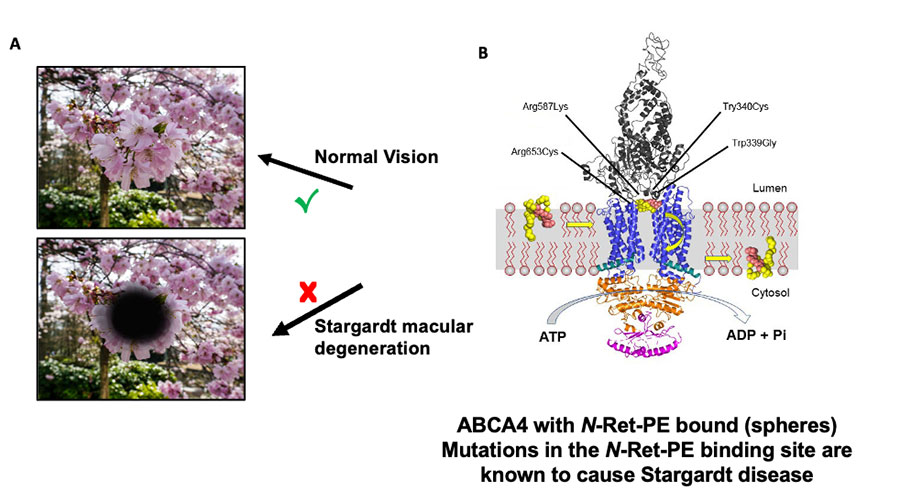
The researchers were interested in finding out how the ABCA4 transporter malfunctions to cause vision loss. They found that a portion of the protein that interacts with N-Ret-PE, known as the binding pocket, is inert in some patients with Stargardt disease. Therefore, the toxic compounds slip out of the ABCA4 transporter and cannot be removed from the retina.
Next, by changing the makeup of ABCA4, the researchers showed they could mimic the effect of the Stargardt mutations.
“We were able to elucidate the mechanism of binding, which paves the way for treatments for Stargardt disease,” Tongzhou Xu, a postdoctoral fellow at UBC and lead author of the study, said.
The team is optimistic that one day there will be a targeted therapeutic for patients with Stargardt disease that may use gene therapy and specialized particles for delivery to the eye. Gene therapy approaches have already been successfully used to correct mutations in a similar transporter, which causes cystic fibrosis.
“We are now applying two types of technologies to alter ABCA4,” Molday said. “One which was developed to specifically correct the DNA with gene-editing approaches. We are coupling that with lipid nanoparticles, which have been used in the COVID-19 vaccine to encapsulate mRNA. So, by combining these two technologies, we envision being able to potentially correct the defects in individuals with Stargardt's disease that have specific point mutations.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Building better tools to decipher the lipidome
Chemical engineer–turned–biophysicist Matthew Mitsche uses curiosity, coding and creativity to tackle lipid biology, uncovering PNPLA3’s role in fatty liver disease and advancing mass spectrometry tools for studying complex lipid systems.

