Peering into ocular waste recycling
A recent study in the Journal of Biological Chemistry revealed the key to a protein that commonly causes blindness. The biological process involves a protein that is essential for transporting toxic compounds out of the eye, similar to a garbage recycling service. The challenge is that, like food and the waste it generates, these compounds are essential for the eye to function properly — until they build up and cause blindness.
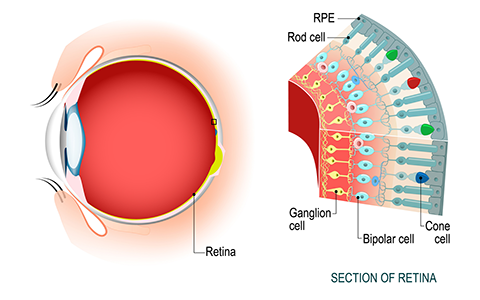
The scientists behind the study research a protein transporter, called ABCA4, that lines the edges of specialized photoreceptor cells in the retina and is normally poised to remove toxic, fatty retinal byproducts called N-Ret-PE. Retinal is a derivative of vitamin A, which is found in foods such as leafy green vegetables.
“Retinal is critical for vision,” said Robert Molday, a professor of biochemistry and molecular biology at the University of British Columbia who oversaw the work. “But, it's also potentially very toxic because it has a very reactive element. So, cells have to be able to balance between using retinal for sustained vision as well as managing its toxicity .”
Mutations in ABCA4 can cause N-Ret-PE buildup, which leads to vision loss in diseases such as Stargardt disease. Stargardt disease is the most common inherited form of macular degeneration and affects approximately 30,000 people nationwide. There is currently no therapy or cure for the disease.
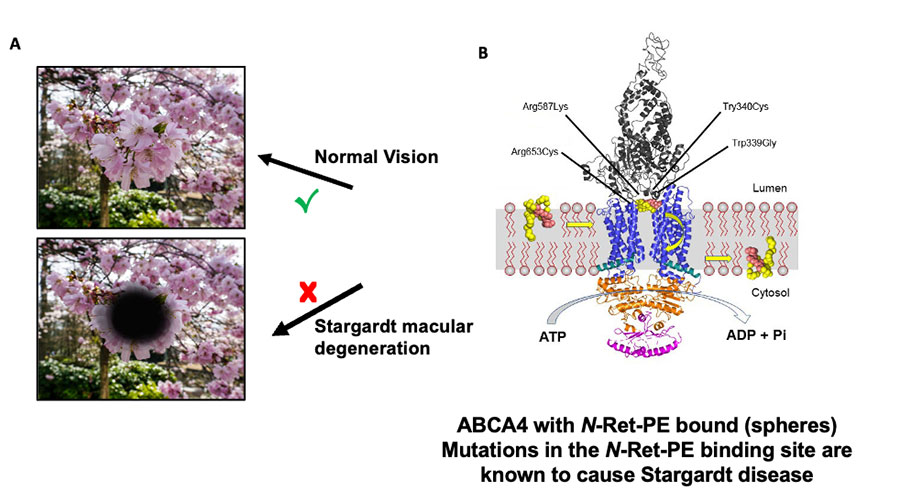
The researchers were interested in finding out how the ABCA4 transporter malfunctions to cause vision loss. They found that a portion of the protein that interacts with N-Ret-PE, known as the binding pocket, is inert in some patients with Stargardt disease. Therefore, the toxic compounds slip out of the ABCA4 transporter and cannot be removed from the retina.
Next, by changing the makeup of ABCA4, the researchers showed they could mimic the effect of the Stargardt mutations.
“We were able to elucidate the mechanism of binding, which paves the way for treatments for Stargardt disease,” Tongzhou Xu, a postdoctoral fellow at UBC and lead author of the study, said.
The team is optimistic that one day there will be a targeted therapeutic for patients with Stargardt disease that may use gene therapy and specialized particles for delivery to the eye. Gene therapy approaches have already been successfully used to correct mutations in a similar transporter, which causes cystic fibrosis.
“We are now applying two types of technologies to alter ABCA4,” Molday said. “One which was developed to specifically correct the DNA with gene-editing approaches. We are coupling that with lipid nanoparticles, which have been used in the COVID-19 vaccine to encapsulate mRNA. So, by combining these two technologies, we envision being able to potentially correct the defects in individuals with Stargardt's disease that have specific point mutations.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

When oncogenes collide in brain development
Researchers at University Medical Center Hamburg, found that elevated oncoprotein levels within the Wnt pathway can disrupt the brain cell extracellular matrix, suggesting a new role for LIN28A in brain development.

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

