JLR: A close-up of the lipids in Niemann–Pick disease
Researchers at the University of Illinois at Chicago have used mass spectrometry imaging to map lipid accumulation in Niemann–Pick disease with unprecedented detail. Their results were published in a recent issue of the Journal of Lipid Research.
There are three major forms of Niemann–Pick disease. All are genetic and rare. Type C, or NPC, results in accumulation of cholesterol and complex lipids known as gangliosides in the endosomes and lysosomes of cells. This accumulation leads to neurodegeneration, killing patients when they are young. Many die before they’re 10. It’s rare for one to live to 40.
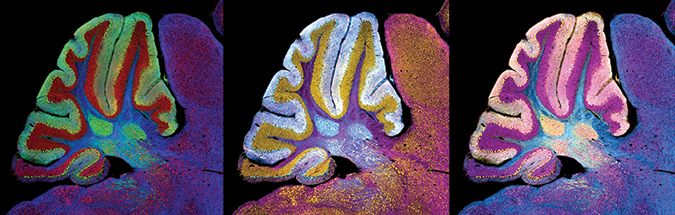 This image of a cerebellum from a mouse with Niemann–Pick C was generated using fluorescence immunolabeling, which is an effective technique for determining protein distribution but cannot capture the location of gangliosides and other lipids that accumulate and cause the disease.Williams/NICHD
This image of a cerebellum from a mouse with Niemann–Pick C was generated using fluorescence immunolabeling, which is an effective technique for determining protein distribution but cannot capture the location of gangliosides and other lipids that accumulate and cause the disease.Williams/NICHD
Based on the way movement and cognition problems emerge in NPC, it seems that different brain regions degenerate at varying stages of the disease. To understand this staging better, it would be useful to visualize lipid accumulation in specific brain regions. This isn’t easy to do with traditional methods, because antibodies against gangliosides are not very specific, so most studies of lipid accumulation in Niemann–Pick disease use homogenized tissue samples from mice with the disease and measure bulk lipids by mass spectrometry.
To achieve greater spatial accuracy, researchers in Stephanie Cologna’s lab used mass spectrometry imaging to look closely at lipids in specific regions of the cerebellum in mice with early-stage NPC. Mass spectrometry imaging, which does not require antibodies or chemical labeling, works by representing small areas of a tissue sample as pixels. The researcher coats a tissue sample in a matrix that helps it to ionize and then collects mass spectra from many tiny areas within that sample.
Each spectrum from one pixel includes information about the abundance of many lipid species. The team used the information about different molecules to make images representing the distribution of lipids across the cerebellum.
Mindful of variations in the intensity of matrix-assisted laser desorption/ionization spectra that can arise from uneven application of the matrix or variability among samples, the team, led by graduate student Fernando Tobias, also devised an algorithm to evaluate the most abundant signals. The algorithm let them filter out noise and compare measurements of wild-type and NPC brain samples more reliably with many replicates.
Once they compared lipid distributions across the cerebellum, the team made the interesting observation that, while two types of ganglioside (GM2 and GM3) are drastically higher in the NPC mouse’s cerebellum, GM1 seems to go up throughout the brain. Also, GM2 elevation is very tightly localized in a part of the cerebellum called lobule X, but it’s not yet clear what that might mean.
The researchers intend to continue using mass spectrometry imaging to get a more granular picture of the disease course.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

