Early lipid changes drive retinal degeneration in Zellweger spectrum disorder
Zellweger spectrum disorder, or ZSD, is a rare genetic disorder that disrupts essential cellular processes in infancy and worsens over time, often leading to blindness and life-threatening complications. Individuals with mild ZSD typically live 30–40 years, though treatment is limited to symptom management. This disorder disrupts essential cellular processes by impairing peroxisomes — small organelles responsible for breaking down toxic substances and producing lipids, which are vital for brain and organ development.
In a recent Journal of Lipid Research study, Samy Omri, a research associate in Nancy Braverman’s lab at McGill University Health Centre, and colleagues focused on mutations in the peroxisomal biogenesis factor 1, or PEX1, gene, the most common cause of ZSD. These mutations disrupt lipid biosynthesis and peroxisome assembly, leading to progressive retinal degeneration — a major cause of childhood blindness in affected individuals.
“Currently, there is no curative treatment, so supportive management aims to alleviate symptoms and improve patients' quality of life,” Omri said. “This includes dietary modifications to address metabolic imbalances, supplementation with essential fatty acids to support neurological function.”
To model ZSD-related retinal degeneration, the researchers used mice with the Pex1-G844D mutation, which mirrors the common human variant.
“The team has been deeply engaged in studying and treating peroxisomal disorders, and this project, investigating the influence of lipid metabolism on retinal health, naturally evolved from that work,” Omri said. “My expertise in retinal physiopathology, along with my interest in early molecular changes preceding inflammation and tissue degeneration, aligns well with the lab's research focus.”
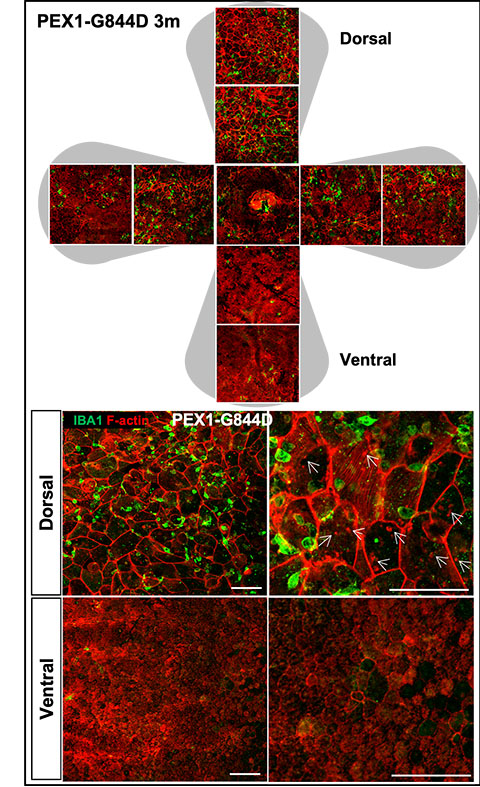
The team focused on retinal pigment epithelium, or RPE, analyzing morphological, inflammatory and lipid changes in a mouse model at one, three and six months of age. They found that RPE degeneration could be detected by three months and worsened with age.
Using mass spectrometry imaging, generated by Pierre Chaurand’s lab, the group identified 47 lipids in RPE that were altered appeared before any visible structural degeneration in the retina. This approach allowed them to visualize the spatial lipid distribution in the retina, providing insight into early disease mechanisms.
“While previous work has largely focused on systemic disease characterization and functional recovery, our research shifts the focus to find new biomarkers for retinal degeneration and a deeper mechanistic understanding of ZSD pathology,” Omri said.
Next, the team plans to validate the clinical relevance of these lipid signatures, a step toward developing future diagnostic tools or therapeutic targets.
They also discovered progressive subretinal macrophage accumulation in ZSD mice, revealing a previously unrecognized inflammatory pathway potentially driving retinal degeneration. Future studies will investigate these inflammatory signal pathways to identify anti-inflammatory therapies that could slow or prevent retinal degeneration.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

