JBC: Iron–sulfur cluster research offers new avenues for investigating disease
Many important proteins in the human body need iron-sulfur clusters, tiny structures made of iron and sulfur atoms, to function correctly. Researchers at the National Cancer Institute, the National Institutes of Health and the University of Kentucky have discovered that disruptions in the construction of iron-sulfur clusters can lead to the buildup of fat droplets in certain cells. These findings, published in the Journal of Biological Chemistry, provide clues about the biochemical causes of such conditions as nonalcoholic fatty liver disease and clear-cell renal carcinoma.
“Iron-sulfur clusters are delicate and susceptible to damage within the cell,” said Daniel Crooks, the postdoctoral fellow who led the new study. “For this reason, the cells in our body are constantly building new iron-sulfur clusters.”
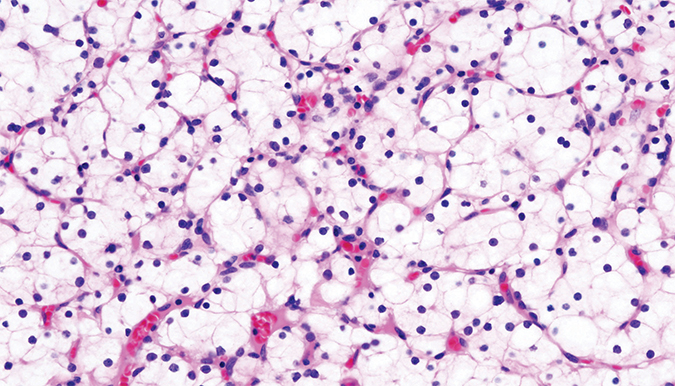 This stained histopathologic image shows clear-cell carcinoma of the kidney from a nephrectomy specimen. The cancer is named for its accumulation of lipid droplets.Courtesy of KGH/Wikimedia Commons Crooks began studying the enzymes that build iron-sulfur clusters during his graduate studies in Tracey Rouault’s lab at the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH. Mutations in one of these enzymes can cause ISCU myopathy, a hereditary condition in which patients, despite seeming strong and healthy, cannot exercise for more than a short time without feeling pain and weakness.
This stained histopathologic image shows clear-cell carcinoma of the kidney from a nephrectomy specimen. The cancer is named for its accumulation of lipid droplets.Courtesy of KGH/Wikimedia Commons Crooks began studying the enzymes that build iron-sulfur clusters during his graduate studies in Tracey Rouault’s lab at the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH. Mutations in one of these enzymes can cause ISCU myopathy, a hereditary condition in which patients, despite seeming strong and healthy, cannot exercise for more than a short time without feeling pain and weakness.
It was clear to Crooks that lifelong deficiency of iron-sulfur clusters caused profound changes in how cells processed energy. But he wondered exactly what happened in a cell in the first moments after something went wrong with iron-sulfur cluster production. Which of the many proteins that need iron-sulfur clusters were affected first, and what effect did this have on cell metabolism?
Crooks developed experimental methods to abruptly stop iron-sulfur clusters from being manufactured in cells and to monitor what happens to how these cells process glucose. Ordinarily, over a series of metabolic steps, cells would convert glucose into energy. But without iron-sulfur clusters, an enzyme called aconitase that carries out one of the steps in this process doesn’t work. As a result, the cells quickly accumulated an intermediate metabolic product called citrate, which eventually was converted into droplets of fat.
Over-accumulation of fats in tissues where they’re not normally found is a hallmark of numerous diseases, including nonalcoholic fatty liver disease, a risk factor for cirrhosis and liver cancer. These findings suggest that this state could be caused by failures of iron-sulfur cluster production — for example due to cellular stressors or toxin exposure.
“We’re hoping that the people who are working so hard on nonalcoholic fatty liver disease will find our paper helpful to their research,” Rouault said.
Crooks, working in the laboratory of surgeon and scientist W. Marston Linehan at the NCI, is now examining the role of iron-sulfur cluster formation and aconitase function in cancers such as clear-cell carcinomas. Various cancers often are characterized by excessive fat accumulation in cells. In fact, this accumulation of lipid droplets is where clear-cell carcinomas get their name: when a slice of such a tumor is fixed on a slide and its proteins are stained, the large areas of lipid accumulation in the cells look transparent.
“We really want to look at the beginnings of cancer,” Crooks said, “… to understand whether the lipids were formed from glucose or other fuels and whether the lipids are important for pathogenesis, or whether they’re just bystanders that form in response to metabolic reprogramming, which is likely to include disruption of iron sulfur protein activities.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

.jpg?lang=en-US&width=300&height=300&ext=.jpg)