From the journals: JBC
We offer a selection of papers on a variety of topics recently published in the Journal of Biological Chemistry. Topics include proteases that fire up the flu, a sulfate pocket to take out MRSA and proteins that prompt cancer protrusions.
Pinpointing the proteases that make flu go viral
There are four main types of influenza viruses — A, B, C and D — and nearly every winter in the U.S. the influenza A and B viruses cause the disease epidemic known as flu season. Globally, influenza A and B account for over 300,000 deaths and 3 million to 5 million cases of severe respiratory illness each year, according to the World Health Organization.
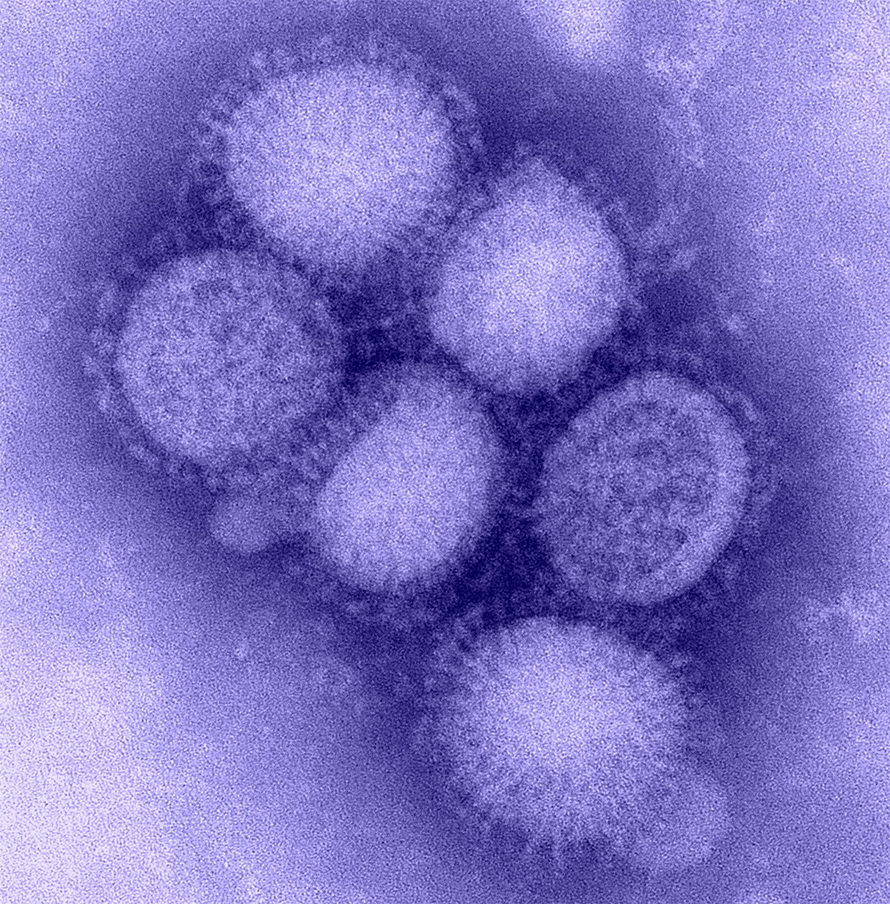
Hemagglutinin, or HA, is a glycoprotein located on the surface of influenza viruses. HA is activated by cleavage by host cell proteases and is essential for initiating the entry of influenza virus into host cells. Understanding the mechanisms of HA protein cleavage is important for development of flu therapies, but the proteases involved in the activation of many viral strains remain unknown.
In a recent paper published in the Journal of Biological Chemistry, Anne Harbig of Philipps-University Marburg and an international team revealed a collection of proteases that process HA and govern protease functionality against specific HA glycoproteins. Using RNA sequencing, infection of mouse cell lines and plaque assay analysis, the authors analyzed the collection of proteases present in mouse lower airway tissues and identified four candidate proteases responsible for influenza A and B HA activation: hepsin, prostasin and two transmembrane serine proteases, or TMPRSSs, known as TMPRSS4 and TMPRSS13.
The researchers used protease inhibitors alongside transfection of TMPRSS13, hepsin and prostasin in double-knockout Tmprss2-/-Tmprss4-/- cells to identify differences in HA reliance on protease cleavage. They found that HA proteases overlap in the two influenza viruses but demonstrate functional differences. Finally, the authors extended their work from mouse airway tissue to human tissue, finding that human orthologs of hepsin and prostasin cleave influenza B HA extensively but not influenza A HA.
The researchers’ data suggest that influenza A and B strains have overlapping sets of proteases that cleave HA differently and that protease activity differs in mice and humans. Identifying the factors responsible for influenza HA activation has both basic science and potential therapeutic applications.
Lab-grown amyloidosis model measures up
Systemic amyloidosis is a rare hereditary disorder that commonly manifests in heart, kidney, liver and nerve complications, sometimes leading to organ failure. Systemic amyloidosis is caused by misfolded proteins, such as transthyretin, resisting the body’s catabolic processes and building up outside cells. Studies seeking to identify the biological mechanisms underlying this condition require clinically relevant disease models.
In recent work published in the Journal of Biological Chemistry, Sara Raimondi of the University of Pavia and an international team compared the characteristics of fibrils generated experimentally with natural transthyretin fibrils to assess their value as a model for systemic amyloidosis. The authors found that the experimentally generated fibrils are thermodynamically and structurally similar to naturally occurring amyloid fibrils.
These results show that laboratory-grown transthyretin fibrils are a useful model for investigating the pathophysiology of amyloidosis.
Sorting sensor domains to unearth MRSA enzyme inhibitors
Infections by methicillin-resistant Staphylococcus aureus, or MRSA, bacteria are notoriously difficult to treat due to the bacteria’s resistance to several antibiotics. Contributing to MRSA’s antibiotic resistance are two integral membrane proteins, BlaR1 and MecR1.
In a recent paper published in the Journal of Biological Chemistry, J. Andrew N. Alexander of the University of British Columbia and colleagues at the University of Maryland and San Francisco General Hospital solved the X-ray crystallographic structures of the BlaR1 and MecR1 sensor domains in complex with avibactam, a beta-lactamase inhibitor that blocks the activity of the beta-lactamase enzyme produced by S. aureus.
These findings reveal the presence of a secondary sulfate-binding pocket that could be exploited in the design of future inhibitors capable of treating MRSA.
Ribosomes don’t rewire protein folding reactions
To function properly, proteins must adopt and maintain specific conformations. They typically reach their final structure by cycling through preferred intermediate conformations, a process that starts during protein synthesis. But how the ribosome affects protein folding remains unclear.
Using a ribosomal force-profiling assay, Madeleine Jensen of the University of California, Berkeley, and collaborators at the University of Cambridge showed that ribosomal interactions have little impact on the folding pathways of RNase H, suggesting that the ribosome does not influence protein folding. Additionally, the authors found that the ribosome promotes RNase H unfolding while the growing protein chain is close to the ribosome, which may limit the detrimental effects of RNase H misfolding and assist in folding fidelity.
These results, reported in a recent paper in the Journal of Biological Chemistry, provide new insights into how ribosomes affect the folding of nascent proteins.
Adaptor protein prompts cancer cell membrane protrusion
Invadopodia — protrusions from the membranes of cancer cells that are rich in the cytoskeletal protein actin — are essential for metastasis. The role of transforming growth factor beta, or TGF-beta, in metastasis has been well established, but how TGF-beta signaling is linked to cancer cell motility as well as its relation to invadopodia remain unknown.
Alex Kiepas of McGill University and collaborators at Laval University revealed that Src-homology/collagen adaptor protein initiates the dynamic adhesion complexes necessary for invadopodium formation in a TGF-beta–dependent manner.
These findings, published in a recent study in the Journal of Biological Chemistry, further researchers’ understanding of cancer metastasis and open new avenues for cancer drug discovery.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

