Tour de flippase
The lipid flippase business is booming. Researchers are discovering new members of this protein family and reporting new structures for old members.
The term “flippase” was coined to describe any protein that catalyzes the flip-flop movement of phospholipid between the two leaflets of a membrane. However, we now recognize three functionally distinct categories of lipid transporters: flippases, floppases and scramblases.
Flippase is used to describe inward-directed pumps that transport lipid unidirectionally from the extracellular leaflet to the cytosolic leaflet, while floppase describes outward-directed pumps that transport lipid in the opposite direction. Most flippases and floppases are adenosine triphosphate–powered pumps in the P4-ATPase and ATP-binding cassette, or ABC, transporter families, respectively. Energy-independent transporters that allow bidirectional transport of lipid are called scramblases, and these are members of several evolutionarily distinct protein families.
Flippases have a primary role in establishing and maintaining membrane asymmetry in eukaryotic cells by enriching phosphatidylserine, or PS, and phosphatidylethanolamine to the cytosolic leaflet of the plasma membrane and removing these lipids from the extracellular leaflet.
The structural basis for lipid substrate recognition by P4-ATPases started to emerge over the past two years from cryo-electron microscopy structures of P4-ATPases with PS bound in an entry site where substrate initially loads. However, recent cryo-EM structures of fungal Dnf1–Lem3 reveal substrate also bound to an exit site that surprisingly sits 10 angstroms above the cytosolic leaflet. It will be interesting to determine if this cytosolically exposed exit site is conserved in other P4-ATPases.
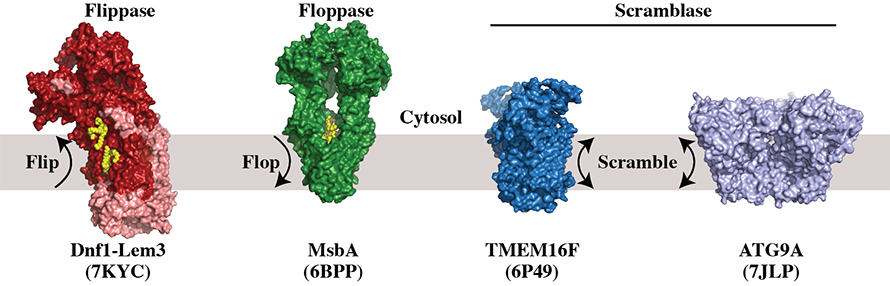
Floppases function in the formation of asymmetric membranes as well as the export of lipids from the cell. MsbA from E. coliis an example of an ABC transporter that flops lipopolysaccharide substrate across the inner membrane. Cryo-EM studies have provided insight into how lipopolysaccharide enters MsbA from the cytosolic leaflet, but how this bulky substrate manages to flop as it moves across the membrane remains unclear.
Two types of scramblases break plasma membrane lipid asymmetry and expose signaling lipids such as PS on the extracellular leaflet. The anoctamin/TMEM16 family of scramblases are activated by a Ca++ influx and expose PS on blood cell membranes to stimulate clotting. Another type of scramblase, Xkr8, primarily acts during apoptosis and is activated by caspase cleavage. This results in PS exposure, which is important for recognition of the cell corpses by phagocytic cells. The Xkr8 structure recently has been reported in a preprint, and it will be fascinating to see how the scrambling mechanism compares between TMEM16F and Xkr8.
Several new scramblases have been discovered with links to autophagy. ATG9, TMEM41B and VMP1, proteins implicated in the growth of autophagosomes, recently were shown to be scramblases. ATG9 localizes to the autophagosome, while VMP1 and TMEM41B proteins are endoplasmic reticulum residents. These scramblases are connected by a lipid transfer protein called ATG2 that mediates movement of phospholipid from the ER to the autophagosome. VMP1 and TMEM41B presumably allow for balanced extraction of lipid from both leaflets of the ER, while ATG9 would allow newly delivered lipid to flow into both leaflets of the autophagosome.
Flippase research is in a bull market right now, and the torrid pace at which new components and mechanistic insights are emerging bodes well for the future of this field.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

