How an enzyme protects the colon
Lipid homeostasis is essential for a person to lead a normal life, and when the human body is unable to regulate lipid metabolism when it is subject to diseases such as metabolic syndromes, inflammation and cancer.

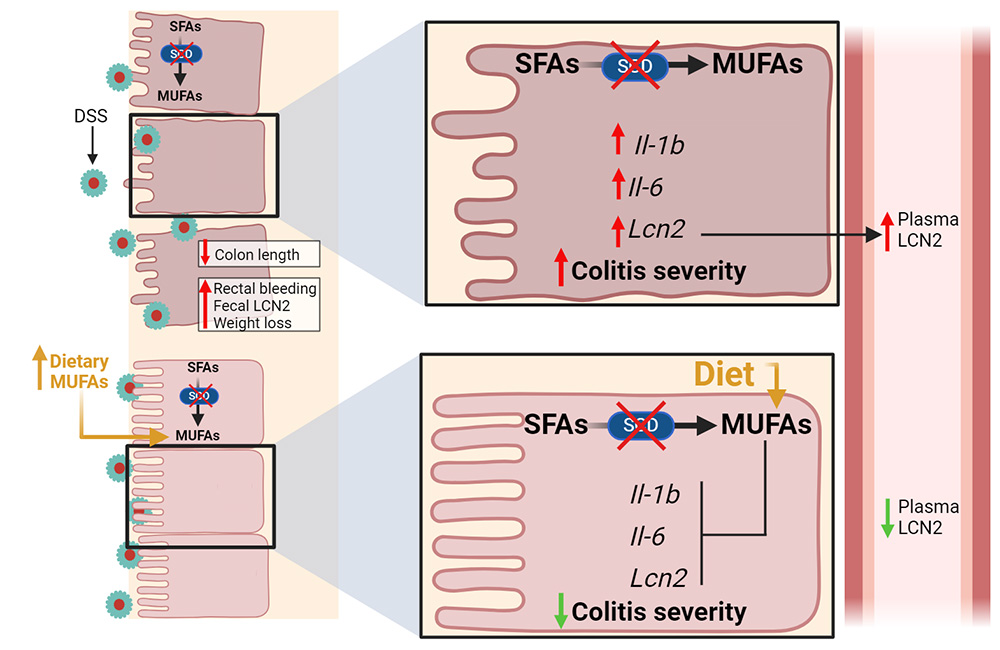
In lipid metabolism, the enzyme stearoyl-CoA desaturase, or SCD, catalyzes the introduction of a 9-cis double bond into saturated acyl chains of palmitoyl-CoA or stearoyl-CoA to produce their monounsaturated forms. These molecules play crucial roles in the synthesis of various tissue lipids.
Expression of SCD is tissue-specific, and while it has been extensively studied in lipogenic tissues, researchers do not yet fully understand its role in the intestines, which are exposed to dietary fatty acids. Recent research shows how intestinal SCD protects against acute colonic inflammation and tissue damage.
Camille Duchamp, a grad student in Harini Sampath’s lab at Rutgers University, and colleagues studied the impact of intestinal SCD on dextran sulfate sodium–induced colitis using mice that had been genetically altered to lack two of the enzyme’s isoforms, SCD1 and SCD2.
“We hypothesize that endogenously synthesized fatty acids may be channeled differentially than those obtained from the diet or circulation,” Duchamp wrote in an email, “such that the absence of the protein that catalyzes their synthesis has acute and profound consequences on cellular and organ health.”
The team assessed fecal blood, weight fluctuations and cytokine levels, and did histological analyses to determine SCD’s influence on disease severity. They saw elevated levels of the protein lipocalin-2, or LCN2, produced by neutrophils, macrophages and intestinal epithelial cells, in the altered mice, indicating a severe colitis phenotype.
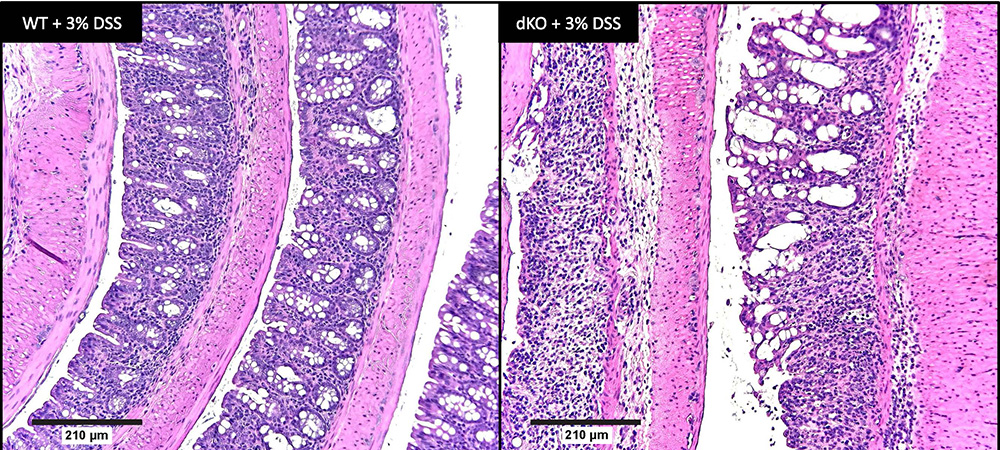
The lab’s previous studies showed that mice lacking just SCD1 had reduced alpha diversity in their intestinal microbiome, a characteristic associated with Crohn’s disease and ulcerative colitis. However, Duchamp noted that deleting SCD1 alone may not significantly impact colitis development, perhaps because SCD2 compensates. By continuing to study the gut microbiome of mice lacking both isoforms, the researchers aim to find potential associations with the increased colonic inflammation in these animals.
Duchamp’s team also found that supplementing the diets of the mice lacking both SCD1 and SCD 2 with monounsaturated oleic acid alleviated colonic inflammation, demonstrating the significance of intestinal lipid composition in health and disease. Despite ample dietary and circulating lipid sources, the mice needed renewed synthesis of monounsaturated fatty acid, or MUFA.
In the lab’s published research, mice lacking intestinal SCD1 exhibited reduced levels of myristoleic acid, an unsaturated fatty acid, prompting speculation that mice lacking both SCD1 and SCD2 may be deficient in additional lipids. In the future, the researchers will use these mice to study how well dietary fatty acid supplements alleviate colitis symptoms and to explore how colonic SCD activity affects development of colorectal cancer.
“Certainly, these studies have high translational potential,” Duchamp wrote. “We are unaware of studies exploring the role of dietary MUFA supplementation in treating colitis symptoms and are excited to extend our work in the mouse model to such translational applications.
Details
Camille Duchamp will present this research from 5:30 to 6:30 p.m. CDT on Sunday, March 24, at Discover BMB 2024, the American Society for Biochemistry and Molecular Biology annual meeting in San Antonio. Her poster is at Board 340.
Abstract title: A role for intestinal stearoyl-CoA desaturase in modulating acute colonic inflammation.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

