Transport of O-palmitoleated Wnts: Where does the lipid go?
Wnts are evolutionarily conserved ligands that signal at short range to regulate morphogenesis, cell fate and stem cell renewal. The first and essential step in Wnts' secretion is their O-palmitoleation by the enzyme porcupine, or PORCN. This lipid modification is unique to Wnts and crucial for limiting their diffusion, restricting Wnt signaling to short range.
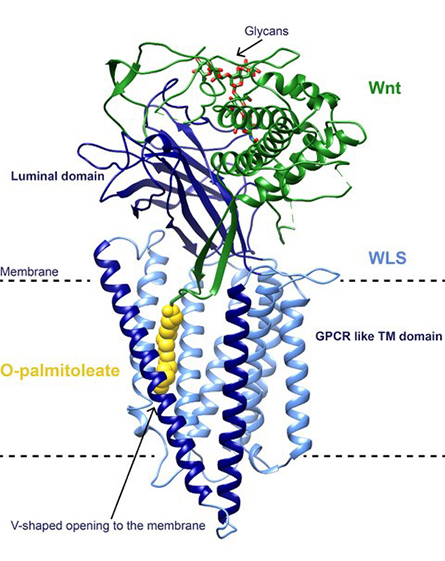
Modification of Wnts has been shown to be essential to their signaling capabilities. After their O-palmitoleation in the endoplasmic reticulum, or ER, Wnts are loaded onto their dedicated transporter Wntless, or WLS, an integral membrane protein with a small soluble domain in the ER lumen. O-palmitoleated Wnts associated with WLS then travel to the plasma membrane, where they are transferred to receptors, such as Frizzled, on the membranes of target cells, in turn triggering the activation of signaling pathways. Structures of Wnt in complex with the cysteine-rich domain, or CRD, of Frizzled have shown how the lipid modification of Wnt is central to binding, with the O-palmitoleate buried deep in a hydrophobic groove of the CRD.
Questions remained about Wnts' transfer from the ER to target cells and the role of the O-palmitoleate: How does the transfer of the O-palmitoleated Wnt from PORCN to WLS occur? Where does the O-palmitoleate reside when Wnts are in complex with WLS, within the protein or in the membrane? How are Wnts released from WLS, and how do they travel with their water-insoluble cargo to neighboring cells?
We recently reported the structure of human O-palmitoleated WNT8A in complex with WLS, determined by single-particle cryo-electron microscopy to 3.2 Å resolution. The structure shows that the WLS membrane domain has close structural homology to G protein–coupled receptors, or GPCRs, with the addition of a transmembrane helix connecting the N-terminus to its luminal domain.
Based on the structures of Wnt bound to the CRD domain of Frizzled, we expected that the O-palmitoleate would be inserted into a hydrophobic binding site within the luminal domain of WLS. Instead, the Wnt hairpin carrying the lipid as a covalent attachment inserts deeply into a conserved hydrophobic cavity in the GPCR-like domain with the O-palmitoleate protruding between two transmembrane helices into the lipid bilayer.
We observed an extensive binding surface between Wnt and WLS, consistent with the known tight interaction between the two, while the energetically favorable environment of the membrane sheltered the hydrophobic cargo. A large opening to the bilayer within the membrane domain of WLS might be the route for shuttling the O-palmitoleate from PORCN to WLS, maintaining the lipid within the bilayer during the transfer and possibly involving a direct interaction between the enzyme and the carrier.
Comparing the structure of Wnt in complex with WLS to the structures of Wnt bound to the CRD domain of Frizzled, we observed a large conformational change on a separate Wnt hairpin, which may be important for its one-way transfer to receiving cells. For this transfer to occur, the O-palmitoleate must be extracted from the lipid bilayer and transferred to the CRD domain of Frizzled.
It's unclear if and when this involves other proteins and whether the transfer occurs in cis (the same) or trans (the opposite) membranes.
This work provides molecular-level insights into a central mechanism in animal body plan development and stem cell biology. We believe it opens up a new direction to explore membrane protein–lipid interactions.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.


