From the journals: JLR
A new way to accelerate wound healing. How pasteurization affects enzymes in human milk. Read about papers on these topics recently published in the Journal of Lipid Research.
A new way to accelerate wound healing
Wound healing is a dynamic process. First the body stimulates clotting factors to reduce blood loss. Then inflammation is initiated to protect wounded tissue from pathogens. After foreign agents are removed, tissue cells proliferate and new epithelial layers form. Many different molecules signal each other in every step of healing, which requires a balance between pro-inflammatory and anti-inflammatory processes. When the signaling cascades and inflammatory balance are impaired, a wound cannot heal.
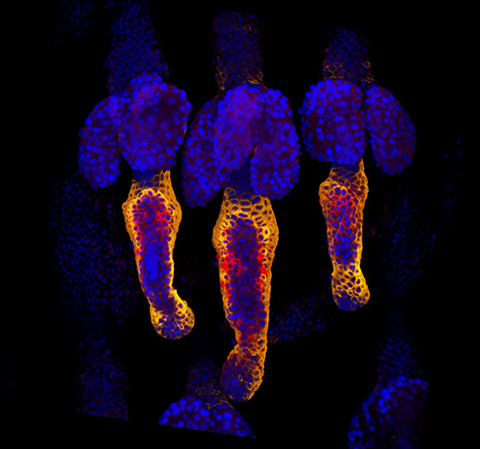
Eicosanoids are signaling lipid molecules that play a role in mammalian wound response and impairment of that response; they include prostaglandins, thromboxanes and leukotrienes. An imbalance between anti-inflammatory eicosanoids, such as prostaglandins, and proinflammatory eicosanoids, such as epoxyeicosatrienoic acids, causes wound healing problems. Thus, researchers study different ways of regulating eicosanoid expression to develop more efficient healing.
Eicosanoids are synthesized by activity of phospholipase A2, or PLA2, which is activated by ceramide-1-phosphate, or C1P — a key regulator of cell growth and survival that plays a crucial role in cell division and DNA synthesis. C1P is regulated by ceramide kinase, or CERK, enzymes, and previous studies have shown that downregulation of CERK blocks PLA2 activation, which causes eicosanoids to be depleted in response to inflammation.
Kenneth D. Maus at the University of South Florida, Tampa, and a team of researchers from the University of Virginia School of Medicine and elsewhere in the U.S. targeted the CERK enzyme by generating small-molecule inhibitors and by genetic manipulation to observe its effect on wound healing. Their article about this work was published recently in the Journal of Lipid Research.
The group developed a small-molecule inhibitor against CERK, and they genetically modified mice to have ablated CERK synthesis. They showed that CERK inhibition or ablation accelerates the transition from inflammation to proliferation in wounds. These results suggest that inhibition of CERK enhances wound healing and maturation of the tissue, and they provide preclinical data to explore future human clinical application.
How pasteurization affects enzymes in human milk
The unique nutritional composition of human milk protects infants from disease by stimulating immune function, establishing the gut microbiome and acting against microbes to reduce infection. However, human milk from a biological parent is not always available. In such cases, experts advise that donated human milk is the best option in the first six months. Before it is fed to an infant, donor milk should be pasteurized to kill bacteria and viruses. Pasteurization has a minimal effect on the nutritional composition of milk; however, exposure to high heat for 30 to 45 minutes may harm some enzymes that are important for lipid digestion.
Triacylglycerols make up 98% of lipid content and provide about 55% of the total energy intake of an infant fed human milk. Digestion of triacylglycerols and subsequent absorption of free fatty acids are essential for brain and nervous system development. However, if pasteurization harms enzymes that play a role in lipid digestion, donor milk is not the best option. Syaza Abu Bakar at the Monash Institute of Pharmaceutical Sciences and a group of researchers in Australia investigated differences in lipid digestion, comparing nonpasteurized and pasteurized human milk, in their recent study published in the Journal of Lipid Research.
Bile salt-stimulated lipase, or BSSL, is an important enzyme for lipid digestion. The researchers observed that nonpasteurized milk has more free fatty acids than pasteurized milk, meaning that pasteurization of milk impairs digestion. Lab experiments showed that adding bile salts to the milk, however, helps to achieve almost complete digestion, suggesting that BSSL is removed in pasteurization. The researchers concluded that, for healthy infant growth, the activities of enzymes such as BSSL should be protected and preserved during pasteurization.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

