JBC: A sugar-attaching enzyme defines colon cancer
Researchers have identified an enzyme that is absent in healthy colon tissue but abundant in colon cancer cells, according to a paper published in the Journal of Biological Chemistry.
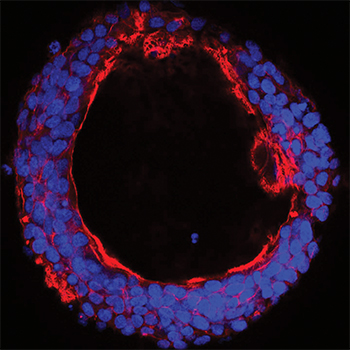 The enzyme GalNAc-T6 is upregulated selectively in colon adenocarcinomas, and its expression is associated with a cancerlike growth pattern.courtesy of Kirstine Lavrsen/University of Copenhagen
The enzyme GalNAc-T6 is upregulated selectively in colon adenocarcinomas, and its expression is associated with a cancerlike growth pattern.courtesy of Kirstine Lavrsen/University of Copenhagen
The enzyme appears to drive the conversion of normal colon tissue into cancer by attaching sugar molecules, or glycans, to certain proteins in the cell. Understanding the role that sugar-modified proteins play in healthy and cancerous cells is an emerging area of cancer biology that may lead to new therapies.
Hans Wandall’s team at the University of Copenhagen studied 20 enzymes that initiate the first step in a particular kind of glycan modification, called GalNAc-type O-glycosylation, found on diverse proteins. These enzymes, called GalNAc transferases, or GalNAc-Ts, are found in different amounts in different tissues, but their functions are poorly understood.
Wandall’s team, led by then-graduate student Kirstine Lavrsen, found that one of the GalNAc-Ts, called GalNAc-T6, was absent in healthy colon tissue but abundant in colon cancer cells. The team used CRISPR/Cas engineering of a colon cancer cell line with and without GalNAc-T6 to understand to which proteins the enzyme helped attach sugars and what effect this had on the cells.
“When we look at the 3D growth of a cancer cell line that has GalNAc-T6, it can form tubular structures with formation of something that looks like colon cancer tissue,” Wandall said. “When we take out GalNAc-T6, then suddenly the tissue formation changes to look more like the crypt structures that you would find in a healthy colon.”
Using mass spectrometry, the team categorized the proteins that GalNAc-T6 acted on in these cells.
“Our data suggest (that) this specific enzyme seems to affect a subset of proteins that could be involved in cell-cell adhesion,” Wandall said. In other words, the glycan modifications changed the patterns in which cells stuck together, leading the cells to develop as something that looked more like a tumor than a healthy tissue.
The next step is to understand precisely why adding sugars to the specific protein sites modified by GalNAc-T6 leads colon cells to develop abnormally. Glycan modifications can affect protein function in myriad ways. For example, they can make proteins that usually are cleaved into two unable to be cleaved, or prevent two proteins from binding to each other.
Wandall hopes that understanding glycosylation in cancer cells will lead to better early diagnostic tools, drugs or immunotherapies.
“Glycans add an additional context layer that could help us create more specific interventions,” he said.
“Glycans look so different in cancer compared to normal tissue, and it’s a really understudied field,” Lavrsen said. “There are a lot of things to be discovered.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

.jpg?lang=en-US&width=300&height=300&ext=.jpg)