Collaboration born at a poster session
It was January 2008.
Lyme disease was — and remains — the most common vector-borne disease in the Northern Hemisphere. Doctors across the United States were beginning to prepare for a summer when nearly 29,000 new cases would be diagnosed.
Celebrex was a blockbuster anti-inflammatory drug, with $2.5 billion in sales that year for treatment of arthritis and acute pain — including the arthritis that can be a complication of untreated Lyme infection.
And in Big Sky, Montana, two graduate students at a Keystone Lipids Conference were cooking up research that would explain why, at least in mice, treating Lyme with Celebrex is a bad idea.
For Victoria Blaho and Matt Buczynski, the conference was the launching pad for an independent career in science. Both came to the meeting as graduate students with well-established projects, looking to expand on a certain angle. Both came away with an exciting new project and, eventually, the answer to a puzzle neither could have cracked alone.
 Victoria Blaho is a research assistant professor at Sanford Burnham Prebys in California.Courtesy of Sanford Burnham Prebys Medical Discovery Institute
Victoria Blaho is a research assistant professor at Sanford Burnham Prebys in California.Courtesy of Sanford Burnham Prebys Medical Discovery Institute  Matt Buczynski is an assistant professor at the School of Neuroscience at Virginia Tech. Courtesy of Virginia Tech
Matt Buczynski is an assistant professor at the School of Neuroscience at Virginia Tech. Courtesy of Virginia Tech
“It’s a great example of why it’s important for graduate students and postdocs to go to meetings,” Blaho said, “especially these small meetings.”
A mouse model of Lyme disease
Lyme disease in humans is caused by infection with the bacteria Borrelia burgdorferi, a spirochete that is transmitted by tick bite. Antibiotics given early can clear the infection. But if left untreated, it can spread to the joints and other tissues, causing arthritis in about 60 percent of cases. In some patients, the arthritis persists even after a course of antibiotics.
 Charles BrownAt the time of the conference, Victoria Blaho was a graduate student studying a mouse model of Lyme infection in Charles Brown’s newly opened lab at the University of Missouri. The group knew that after being infected with B. burgdorferi in the hind paw, their mice developed arthritis in the nearest ankle joint, mediated by the innate immune system. The arthritis resolved as the immune system cleared the bacteria from the joints. While studying this model, Blaho had found that treating mice with Celebrex could prevent their arthritis from healing.
Charles BrownAt the time of the conference, Victoria Blaho was a graduate student studying a mouse model of Lyme infection in Charles Brown’s newly opened lab at the University of Missouri. The group knew that after being infected with B. burgdorferi in the hind paw, their mice developed arthritis in the nearest ankle joint, mediated by the innate immune system. The arthritis resolved as the immune system cleared the bacteria from the joints. While studying this model, Blaho had found that treating mice with Celebrex could prevent their arthritis from healing.
“It didn’t make the disease worse,” she said about the drug. “But it didn’t decrease the inflammation, and it prevented the ability of the arthritis to resolve.” Celebrex inhibits a specific cyclooxygenase enzyme, Cox-2, which converts arachidonic acid released from the cell membranes by the phospholipase A2 enzyme, or PLA2, into lipid mediators of inflammation. So it was surprising that inhibiting the Cox-2 enzyme would turn a short inflammatory response to infection into a chronic inflammatory disease.
“It’s exactly the opposite of what you’d observe in other models,” Blaho’s graduate mentor, Charles Brown, said.
In other mouse models of arthritis, which are driven by an antibody response to the mouse’s own tissue, inhibiting Cox-2 prevents joint pain. But unlike most mouse models, the Lyme disease model involved a whole pathogen, rather than a single molecular component, and a systemic immune response to it. The lab knew that the drug did not affect development of an antibody response compared to untreated animals, or clearance of Lyme bacteria from the joint where Blaho had introduced it.
To understand how the drug changed the course of infection, Blaho and Brown reasoned that they needed to understand how Cox-2 products change during infection. But the compounds are difficult to extract and study; at the time, no commercial services offered lipidomic assays, and they didn’t know anyone in the lipid field.
A novel technique and a felicitous meeting
Matt Buczynski, a student of lipid biochemist Edward Dennis at the University of California, San Diego, was at the forefront of the new wave of lipidomics. He had been second author on a recent paper out of the Dennis lab that introduced a new technique for simultaneously identifying and quantifying up to 60 lipid species in the eicosanoid family. Eicosanoids are water-soluble mediators of inflammation that all are derived from arachidonic acid released from the cell membrane by PLA2 and then modified by various enzymes, including Cox-2.
 Edward Dennis The eicosanoids have various biological effects, but because they are so similar, each representing a different minor modification to arachidonic acid, it was difficult to tell them apart. Being able to measure them separately was an important advance. The lab had piloted the development of the lipidomics field by exploiting a cell model of inflammation.
Edward Dennis The eicosanoids have various biological effects, but because they are so similar, each representing a different minor modification to arachidonic acid, it was difficult to tell them apart. Being able to measure them separately was an important advance. The lab had piloted the development of the lipidomics field by exploiting a cell model of inflammation.
“We were working with macrophages, and doing these really cool lipidomics approaches,” Buczynski said. “B ut of course if you’re working with one cell type, you’re only seeing part of the picture.”
When she first encountered his work, Blaho appeared to feel that way. Buczynski recalls sitting in the audience at the start of the 2008 Keystone Conference on Eicosanoids and Other Mediators of Chronic Inflammation as Dennis gave the plenary lecture. Buczynski introduced himself to the graduate student sitting next to him, Blaho, who didn’t know his connection to the speaker.
“She was like, ‘This guy’s only looking at one cell type; what does he think he’s going to find? This is really cool stuff, but he’s got to look at better models,’” Buczynski said.
With a chuckle, he recalls the look on her face when Dennis concluded the talk with an acknowledgments slide and Buczynski’s picture.
“She just looked over at me like, ‘Ohhhhh, man.’”
Fortunately, Buczynski wasn’t offended.
At the poster session after the plenary lecture, where posters were arranged alphabetically, the two found themselves next to one another once again.
“It gave us a great opportunity to talk about the really cool mass spec approaches that we were using and the really amazing model that she had developed,” Buczynski said.
Between them, they realized that the two projects were perfectly complementary.
Blaho had seen a change in lipid-mediated inflammation but had no way to measure the change in lipids. Buczynski had a technique for measuring changes to many lipids at once but was in search of an interesting question to attack with it.
“It was a very propitious time,” Blaho said of the meeting. “It just so happened that Matt really wanted to get some sort of in vivo relevance and I really needed someone who had the biochemical expertise for measuring these (lipids).”
Cooking up a collaboration
Over the rest of the conference, the pair fleshed out a plan to work together.
“It was scientific, but it was also building a rapport,” Buczynski said of their ongoing conversation. “That’s one of the things that makes conferences so valuable. You can read about someone’s work online, but until you meet them, it’s hard to tell if this is someone that you could really work with for the next one to three years.”
Back in the lab, it took time to work out how to extract lipids from the hard tissue of the mouse ankle and ship them from Missouri to California unharmed. Methodology is often a hurdle for lipid biologists, Blaho said.
“Despite the fact that we’ve known about these things for decades and decades, there’s still so much we don’t know,” she said, “and so much we have to do on our own, for experimental protocols.”
While Brown had approved the pilot experiments, the Dennis lab was already busy with quite a few collaborations. This one, with its untested tissue extraction protocols, was risky. So Buczynski decided to run a few covert pilot experiments to see if the idea could be fruitful.
“We had just started running a lot of samples from lots of different collaborators, and so sneaking in one or two pilot samples wasn’t too challenging,” he said. “It was important, because you don’t want to invest a lot of money doing this elaborate time course if there’s literally nothing in the sample.”
With the technical difficulties sorted out, the pair presented their preliminary data to both PIs. They made a strong case that they could track eicosanoids from arthritic joints over time and that the collaboration was worth investing in. In April, just four months after their first meeting, they presented a preliminary lipid time course in B. burgdorferi-infected wild type mice at the 2008 American Society for Biochemistry and Molecular Biology Annual Meeting in San Diego. They published their work in the ASBMB’s Journal of Biological Chemistry in August 2009.
A curiosity-driven project
Dennis called the mouse model “a beautiful model of an infection and its resolution.” After being infected, the mice have a dramatic, localized inflammatory response as B. burgdorferi invades the ankle. The joint swells up, becoming infiltrated with innate immune cells, and the mice start to show symptoms of arthritis. As the adaptive immune system comes to the rescue, generating antibodies against B. burgdorferi, the bacterium is cleared, after which the arthritis generally resolves.
Because Blaho had found that inhibiting Cox-2 prevented resolution of arthritis, the grad students compared lipid profiles in two groups of animals: one with and one without the Cox-2 gene.
“We measured as many different (lipid metabolites) as possible, regardless of whether we had any idea whether they were involved,” Blaho said. “It was kind of our version of shotgun lipidomics.”
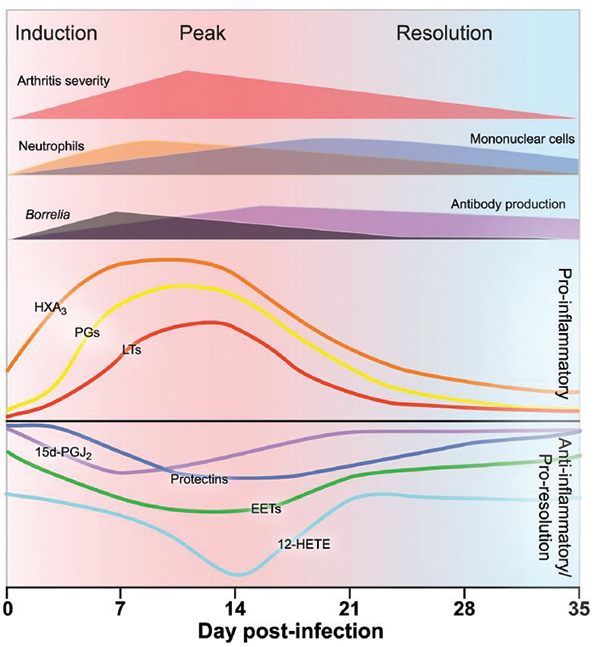 A figure from Victoria Blaho and Matt Buczynski’s 2009 paper in JBC summarizes the production of pro-inflammatory and anti-inflammatory signaling lipids over the course of infection in wild-type mice infected with Borrelia burgdorferi. Courtesy of Victoria Blaho and Matt Buczynski
A figure from Victoria Blaho and Matt Buczynski’s 2009 paper in JBC summarizes the production of pro-inflammatory and anti-inflammatory signaling lipids over the course of infection in wild-type mice infected with Borrelia burgdorferi. Courtesy of Victoria Blaho and Matt Buczynski
The two graduate students spent hours on the phone, each looking at the same spreadsheets, searching for differences between the Cox-2-deficient mice and the untreated controls.
“We could see, corresponding with the peak of infection, the production of all of these bad prostaglandins that cause inflammation and swelling,” Dennis said of the wild-type controls.
“Then after a small time delay, we could see the production of protectin.”Prostaglandins are eicosanoid products of Cox-2; protectin, which signals for resolution of inflammation, is another lipid made by the enzyme 5-Lox. Strangely, in the mice with a knockout of Cox-2, there were not only lower levels of the eicosanoid products of Cox-2 products but also lower 5-Lox products.
Inhibiting Cox-2, and thereby reducing the circulating prostaglandin eicosanoids, would have been expected to stop inflammation in its tracks. But Blaho and Buczynski’s findings reflect a role for Cox-2 in the resolution of inflammation. Some of its products are potent anti-inflammatory molecules, and others are precursors to production of pro-resolution molecules, such as protectin, by other enzymes. When mice were treated with Celebrex to inhibit Cox-2, or never had Cox-2 to begin with, the protectins never were made; therefore, the inflammatory response continued indefinitely.
The work contributed to a growing sense in the field that resolution of inflammation is an active process rather than a vague fizzling out of ongoing pro-inflammation signals.
Two careers launched
When they left graduate school, Buczynski and Blaho moved on from the mouse Lyme problem to different questions about the biological roles of lipids, but it isn’t hard to see interests that they described in this project reflected in their current research programs. Both have become assistant professors in the last two years.
Following an interest in neuroscience derived from another graduate school collaboration, Buczynski joined the lab of the late Larry Parsons at The Scripps Research Institute as a postdoc. He studied behavioral and lipidomic changes that happen during nicotine addiction, work he has continued since starting his own lab as an assistant professor at Virginia Tech in 2016.
Blaho, who started graduate school as an immunologist, has continued to surf the intersection of lipid biochemistry and inflammation. She went to Weill Cornell Medical College to study sphingosine-1 phosphate, or S1P, as a postdoc with Timothy Hla and now works on S1P signaling in the immune system as a research assistant professor at Sanford Burnham Prebys Research Institute in La Jolla, California.
Dennis and Brown have continued to collaborate on eicosanoids in Lyme disease, co-authoring four subsequent research articles and a review last year on the model system.
 Borrelia burgdorferi, the bacterium behind Lyme disease in humans, does not usually cause symptoms in infected mice. Blaho and Buczynski worked in the C3H mouse line, which is unusually susceptible to borreliosis and arthritis. Courtesy of Tina Carvalho, University of Hawaii at Manoa “We’re still trying to understand how the innate immune system recognizes Borrelia and how that leads to development of disease,” Brown said. “When does the immune system decide that it’s time to resolve? What are those signals for resolution?”
Borrelia burgdorferi, the bacterium behind Lyme disease in humans, does not usually cause symptoms in infected mice. Blaho and Buczynski worked in the C3H mouse line, which is unusually susceptible to borreliosis and arthritis. Courtesy of Tina Carvalho, University of Hawaii at Manoa “We’re still trying to understand how the innate immune system recognizes Borrelia and how that leads to development of disease,” Brown said. “When does the immune system decide that it’s time to resolve? What are those signals for resolution?”
Meanwhile, Dennis has expanded his lab’s focus to understand the role lipid signaling plays in other infections, lately adding an influenza angle.
“In patients, we identified numerous eicosanoids that increased with influenza, including both pro-inflammatory prostaglandins and anti-inflammatory (pro-resolution) eicosanoids,” he said. “So lipidomics has led to identifying new therapeutic approaches to infectious disease.”
Medical guidelines for Lyme disease do not suggest non-steroidal anti-inflammatory drugs, also known as NSAIDs. But they also don’t contraindicate them. “That paper would suggest that for people diagnosed with Lyme disease, it would be prudent not to take NSAIDs,” Brown said. “I’m not sure that the clinicians are paying attention to that part.”
Physician-scientist Linda Bockenstedt of Yale University, a clinician whose lab studies possible mechanisms for lasting Lyme-related arthritis, sees it differently.
 Linda Bockenstedt “We don’t have any evidence in humans that taking anti-inflammatories — steroids, or NSAIDs, or selective Cox inhibitors — is detrimental after antibiotics have been started,” Bockenstedt said. “Obviously, with an infection, we would like to eliminate the cause of the inflammation first, before giving drugs to suppress the inflammation.”
Linda Bockenstedt “We don’t have any evidence in humans that taking anti-inflammatories — steroids, or NSAIDs, or selective Cox inhibitors — is detrimental after antibiotics have been started,” Bockenstedt said. “Obviously, with an infection, we would like to eliminate the cause of the inflammation first, before giving drugs to suppress the inflammation.”
She adds that anecdotes from scattered patients link steroid injections, administered to joints to alleviate arthritis before the start of antibiotics, with antibiotic-refractory arthritis. However, starting antibiotics is the standard of care, and there never has been a controlled study of steroids to see if they cause the complications.
Though their research interests have taken them in different directions, Blaho and Buczynski keep in touch. They both have fond memories of the the collaboration and the sense of community it gave them.
“Having the ability to talk to people who you know are experts and will be interested makes such a difference,” Blaho said, “versus a shot in the dark and people who don’t know what’s important or exciting or worth funding.”
Doing the study together “made me realize how much more fun it is to collaborate,” Buczynski said. “I don’t feel like there’s anything I’ve accomplished in science that didn’t involve other people’s help, and it’s more fun when they feel like they got kudos for that.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

