From the journals: MCP
We provide summaries of recent papers published in the journal Molecular & Cellular Proteomics. This month's highlighted topics include using silver nanoparticles to target cancer, glycosylation of the SARS-CoV-2 spike protein and the glycan signature in colorectal cancer tumors.
Silver nanoparticles target metabolically unadaptive cancer cells
Silver nanoparticles have many applications, including medical uses such as wound dressings and implants, because of their antimicrobial activity: They disrupt cell wall integrity and perforate cell membranes. The particles are considered nontoxic to humans, but researchers have observed that they sometimes cause oxidative stress and programmed cell death by disrupting mitochondrial membranes. These observations raise concern about occupational exposure to silver nanoparticles — but also suggest they might make promising anticancer therapeutics, since cancer cells tend to be more metabolically active than their healthy counterparts.
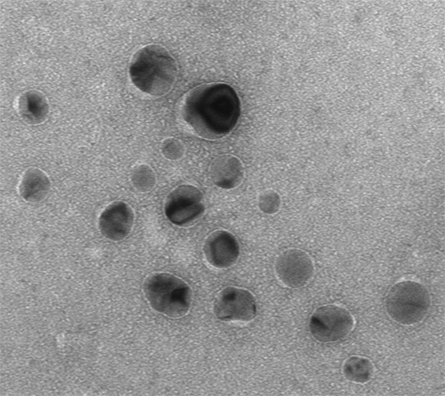
as a cancer treatment at the Wake Forest School of Medicine.
In a recent article in the journal Molecular & Cellular Proteomics, Reetta Holmila and colleagues based at Wake Forest School of Medicine's Center for Redox Biology and Medicine report on an investigation into silver nanoparticles as a cancer treatment. The team used redox proteomics, which detects both the abundance of proteins and their oxidation at cysteine residues, to investigate nanoparticle responses of two cancer cell lines from lung epithelia. Previously, the team had established that one line was sensitive to silver nanoparticle treatment, proliferating more slowly or dying after exposure, while the second was resistant.
In the new work, they observed that protein oxidation increased in both cell types in the hours after silver nanoparticles were introduced. The resistant cells mounted a robust oxidative stress response through metabolic adaptation, upregulating protein turnover and antioxidant synthesis pathways. As a result, they tended to show less protein oxidation after a long treatment with silver particles than after a short one. The more sensitive cell line failed to ramp up antioxidant metabolism and mitochondrial protection as strongly, and it maintained high oxidation levels, which was ultimately detrimental to cells.
Using microscopy, the researchers observed that the sensitive cell line had started out with mitochondria that were larger and less morphologically defined than their counterparts'. After nanoparticle treatment, mitochondria in sensitive cells swelled and sometimes burst. The findings suggest that preexisting mitochondrial abnormality might predispose some cancer cell lines to respond to nanoparticle treatment by dying, while others manage to adapt.
Glycosylation of the SARS-CoV-2 spike protein
Severe acute respiratory syndrome coronavirus-2, or SARS-CoV-2, the virus responsible for the ongoing COVID-19 pandemic, is known to be glycosylated — that is, infected cells attach sugar molecules to viral proteins during replication. In the most common form of glycosylation, known as N-glycosylation, sugars are attached to the amino acid asparagine. The location and composition of these attached sugars can obstruct protein–protein interactions, thereby modulating processes like infectivity of and human immune reaction to a virus.
In a recent article in the journal Molecular & Cellular Proteomics, Yong Zhang, Wanjun Zhao and colleagues at Sichuan University describe how they characterized N-glycosylation of the SARS-CoV-2 spike protein, which mediates viral entry into human cells. Using mass spectrometry, the team identified 22 sites of N-glycosylation in the virus' spike protein. The composition of the attached sugars is heterogeneous and depends on the host cell, not the glycosylation site: Insect and human cells preferentially attach high-mannose and complex sugars, respectively. Given the influential role of glycosylation on viral infectivity and immunogenicity, these findings will inform future therapy and vaccine development for COVID-19.
Characterizing the glycan signature of tumor tissue
Despite vast improvements in cancer diagnosis and care in recent decades, clinicians still struggle to predict which patients will and will not respond to a specific therapy, which can lead to avoidable disease progression and death. Fanny Boyaval of Leiden University and a team of researchers in the Netherlands hypothesized that the N-glycan signature in tumor tissues can predict patient outcomes. N-glycans, or sugar molecules attached to the surface of membrane-bound and secreted proteins, regulate cancer-related processes including angiogenesis, immunity, metastasis, tumor cell invasion and cell–cell signaling.
Boyaval and her colleagues characterized biopsies from stage II colorectal cancer patients using matrix-assisted laser desorption/ionization mass spectrometry imaging, or MALDI-MSI, a technique that produces a spatially resolved picture of N-glycan distribution and composition in a tissue sample. Writing in the journal Molecular & Cellular Proteomics, they report that cancer cells exhibit an increase in sialylated and high-mannose glycans and a decrease in fucosylated and highly branched glycans, but these changes do not correlate with patient survival. However, the N-glycan signature of tumor-adjacent tissue is similar to that of cancer cells and correlates with patient survival. The study shows that MALDI-MSI can characterize primary tissue samples and provides a new technique to classify colorectal cancer patients.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.


