A second chance for a healthy heart
A recent study using mice has revealed a way to turn back the clock after heart attack. The researchers behind the work used RNAs to instruct cells in an injured heart to eliminate scar tissue and re-create cardiac muscle, allowing the heart to function like new again.
Cardiovascular disease, including heart attack, is the leading cause of death worldwide.
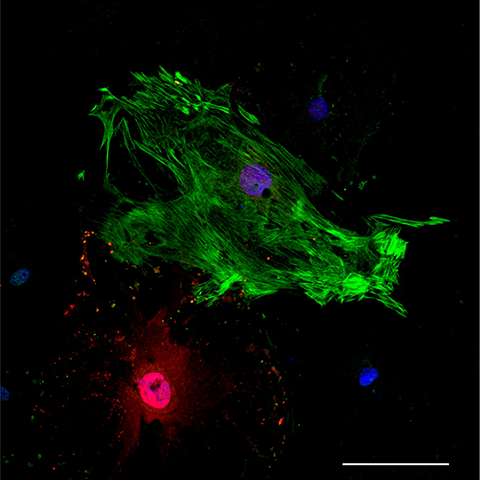
“Adult human hearts are not very good at repairing themselves,” said Conrad Hodgkinson, an associate professor of medicine and pathology at Duke University School of Medicine who oversaw the study. “Once they have a heart attack or any type of damage, there's no capacity to replace the heart muscle that dies. So, what the heart does to stop itself from basically blowing up is it activates fibroblasts to come in and form a scar.”
Like the scars on skin that result from injury or surgery, the scar tissue generated in the heart after a heart attack is tough and nonflexible and can prevent the organ from functioning at its full potential, Hodgkinson said.
Hodgkinson and his team wanted to find an efficient way to convert the scar tissue back into functioning cardiac muscle to essentially reverse the effects of a heart attack. To do this, they set out to find a way to transform fibroblasts, a type of cell that contributes to the formation of connective tissue, into heart muscle cells via a process called cellular reprogramming. Hodgkinson’s lab delivers reprogramming instructions to cells in form of RNAs. However, they found adult fibroblasts are not very good at following the instructions and are resistant to reprogramming.
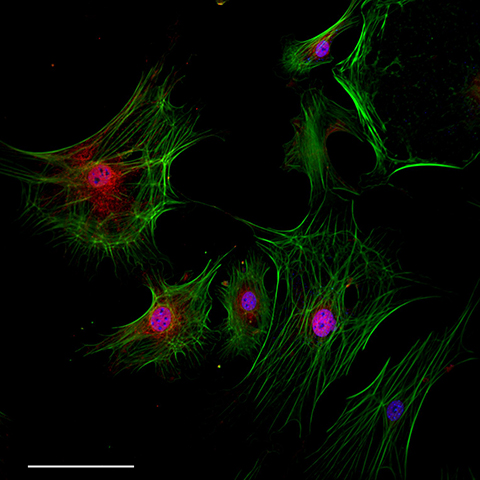
An old adage states: Kids are resilient. However, there may be biology to back it up.
“We found that if you take cardiac fibroblasts from juveniles, they reprogram very nicely,” Hodgkinson said. “But, if you take cardiac fibroblasts from adults, they don't, in fact, respond at all. So, we tried to understand whether the aging process was actually interfering with fibroblast reprogramming.”
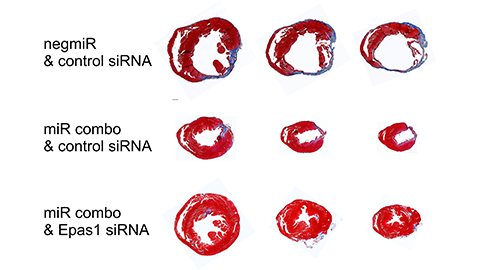
Hodgkinson and his team discovered that a protein oxygen sensor, Epas1, prevents adult fibroblasts from reprogramming themselves. The researchers were able to harness the regenerative capacity of young cells by blocking Epas1 in adult fibroblasts.
“When we reversed the fibroblast aging process, essentially making the fibroblasts think they were young again, we converted more fibroblasts into cardiac muscle,” Hodgkinson said.
The researchers formulated a cocktail of RNAs and packaged them into exosomes, a natural product produced by most cells. This technology allowed them to deliver the exosomes without surgical interventions. Exosome packages have unique properties that guide them to cardiac fibroblasts inside an injured heart.
“Exosomes are kind of like shopping bags,” Hodgkinson said. “The cell sticks a lot of stuff into a big fat ball to send out and signal to other cells. They are a way cells can talk to each other.”
When the researchers used the RNA-filled exosomes to instruct the fibroblasts to reprogram themselves in a mouse that had just experienced a heart attack, the results were, according to Hodgkinson, “impressive.”
“We were able to recover almost all of the cardiac function that was lost after a heart attack by reversing the aging of the fibroblasts in the heart,” Hodgkinson said. Their findings were published in the Journal of Biological Chemistry.
Cellular reprogramming, coupled with reversing cellular aging, has limitless future applications, including restoring neuron loss in the brains of dementia patients and eliminating skin scarring in psoriasis patients without invasive surgical interventions, Hodgkinson said.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

