Dysregulation of a lipid transfer protein linked to brain disorders
Advanced studies of human genetics are a big wave in the medical sciences. Collaborative teams of clinical geneticists and bioinformaticians are surfing this wave, rapidly discovering genomic variations associated with specific human disorders. This trend is providing scientific bases for personalized medicines but also new, important questions linked to the basic biochemistry field.
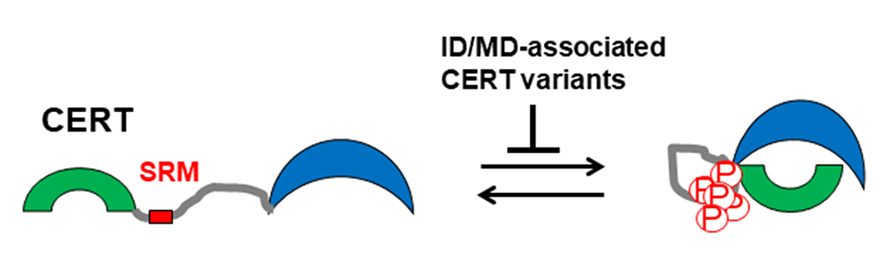
Ceramide transport protein, or CERT, moves the waxy lipids known as ceramides in cells for the synthesis of sphingomyelin, a membrane lipid that is ubiquitous in mammalian cells. In 2007, researchers found that CERT is functionally repressed by multiple phosphorylations of a serine-repeat motif, or SRM, in CERT. At the time, scientists regarded this finding as pure biochemistry of a protein.
However, a decade later, large-scale human genetic studies on intellectual disabilities and mental development disorders, or ID/MD, showed that missense mutations in or near the CERT SRM-encoding regions are associated with a type of autosomal dominant hereditary ID/MD. The dominant inheritance was in line with a prediction from the previous biochemical study that loss of hyperphosphorylation of the SRM renders CERT abnormally active.
Our recent collaborative study confirmed this prediction by demonstrating that substitution of a serine residue in the SRM with other residues similar to variants found in ID/MD patients results in dysregulation of CERT in cultured cells. Nonetheless, several ID/MD-associated missense mutations that occurred in the CERT gene CERT1 also are mapped outside the SRM. This riddle was answered by another recent study showing that a non-SRM variant also compromises the SRM hyperphosphorylation, thereby abnormally activating CERT.
Moreover, cell biological analysis showed that abnormally activated CERT mutants exhibit an aberrant punctate distribution in cells, suggesting that the subcellular distribution pattern is applicable as a diagnostic tool to assess whether a CERT1 variant is an abnormally activated type that may cause ID/MD, although the precise identity of the puncta structure remains undetermined.
Want more lipid research news?
Check out Lipid Trends, a curated collection of hot picks from the world of lipid research, brought to you by LIPID MAPS.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

