From the journals: JLR
Leveraging TRIM38 in fatty liver disease progression and “no eating after bedtime.” Read about these papers recently published in the Journal of Lipid Research.
Leveraging TRIM38 in fatty liver disease progression
Non-alcoholic fatty liver disease, or NAFLD, affects more than a quarter of the world’s adult population. Patients with NAFLD can be asymptomatic or present with insulin resistance, fatigue and abdominal pain. NAFL, or simple steatosis, can evolve into nonalcoholic steatohepatitis, or NASH, cirrhosis, and, in some cases, liver cancer. Yet, no established pharmacological therapy exists for NASH.
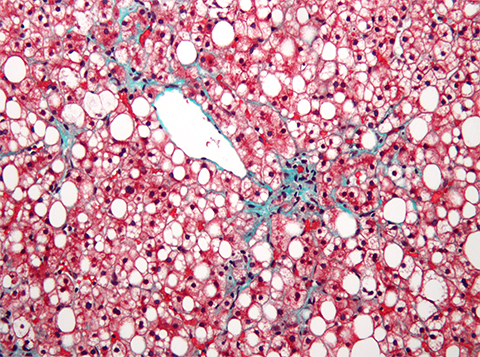
In a recent study published in the Journal of Lipid Research, Xinxin Yao, Ruixiang Dong and colleagues from Taikang Medical School in Wuhan University and other research centers in China explored the potential of tripartite motif 38 protein, TRIM38, as a therapeutic approach to treat NAFLD and NASH.
TRIM38 is part of a superfamily of proteins with regulatory functions over the immune system and the inflammatory response. Specifically, TRIM38 prevents the activation of nuclear factor kappa-light-chain-enhancer of activated B cells, better known as NF-kB, a mediator of inflammation in mammalian tissues that can play a role in the pathogenesis of NAFLD.
The authors found that TRIM38 was downregulated in liver samples from human patients with NAFLD and that deleting TRIM38 in mice worsened high-fat diet–induced hepatic steatosis, inflammation and fibrosis. To confirm the role of TRIM38 in liver disease, the researchers overexpressed the protein in cultured hepatocytes and then exposed them to high concentrations of lipids in the culture media.
The study showed that TRIM38 suppresses expression of inflammation-related and lipid anabolism genes. These findings position TRIM38 as a promising therapeutic ally to help alleviate NAFLD and prevent progression towards NASH.
‘No eating after bedtime’
Do you enjoy late dinners and midnight snacks? You might want to reconsider. Studies have shown a positive correlation between night eating and obesity; that is, the later in the day you consume calories, the greater your chances of gaining weight.
Wenhao Ge, Qi Sun and colleagues at the Nanjing University of Science and Technology in China study the mechanism behind time-delayed eating patterns and body fat accumulation in mice. Their recent results were published in the Journal of Lipid Research.
The authors found that dietary oil is preferentially incorporated into triglycerides and accumulated in adipocytes, or fat cells, when consumed at night rather than during the day.
In mammals, biologically determined rhythms, also known as the circadian clock, control feeding behavior and feeding-related processes in organs and tissues. This study found that the circadian protein Period 1, or Per1, directly contributes to night eating–associated obesity by modulating the activity of two key enzymes responsible for hepatic bile acid production.
In the gut, bile acids are necessary for correct emulsification and absorption of fat. Accordingly, mice lacking Per1 could not absorb fat during night feeding and were thus resistant to high-fat diet–induced obesity. When the researchers treated mice lacking Per1 with cholic acid, one of the most abundant acids in bile, intestinal fat absorption and accumulation in the adipose tissue were partially restored.
This study suggests that Per1 could be a potential target in treating obesity.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

