Plants get a GMO glow-up
As any avid gardener will tell you, plants with sharp thorns and prickles can leave you looking like you’ve had a run-in with an angry cat. Wouldn’t it be nice to rid plants of their prickles entirely but keep the tasty fruits and beautiful flowers?

I’m a geneticist who, along with my colleagues, recently discovered the gene that accounts for prickliness across a variety of plants, including roses, eggplants and even some species of grasses. Genetically tailored, smooth-stemmed plants may eventually arrive at a garden center near you.
Acceleration of nature
Plants and other organisms evolve naturally over time. When random changes to their DNA, called mutations, enhance survival, they get passed on to offspring. For thousands of years, plant breeders have taken advantage of these variations to create high-yielding crop varieties.
In 1983, the first genetically modified organisms, or GMOs, appeared in agriculture. Golden rice, engineered to combat vitamin A deficiency, and pest-resistant corn are just a couple of examples of how genetic modification has been used to enhance crop plants.
Two recent developments have changed the landscape further. The advent of gene editing using a technique known as CRISPR has made it possible to modify plant traits more easily and quickly. If the genome of an organism were a book, CRISPR-based gene editing is akin to adding or removing a sentence here or there.
This tool, combined with the increasing ease with which scientists can sequence an organism’s complete collection of DNA – or genome – is rapidly accelerating the ability to predictably engineer an organism’s traits.
By identifying a key gene that controls prickles in eggplants, our team was able to use gene editing to mutate the same gene in other prickly species, yielding smooth, prickle-free plants. In addition to eggplants, we got rid of prickles in a desert-adapted wild plant species with edible raisin-like fruits.
We also used a virus to silence the expression of a closely related gene in roses, yielding a rose without thorns.
In natural settings, prickles defend plants against grazing herbivores. But under cultivation, edited plants would be easier to handle – and after harvest, fruit damage would be reduced. It’s worth noting that prickle-free plants still retain other defenses, such as their chemical-laden epidermal hairs called trichomes that deter insect pests.
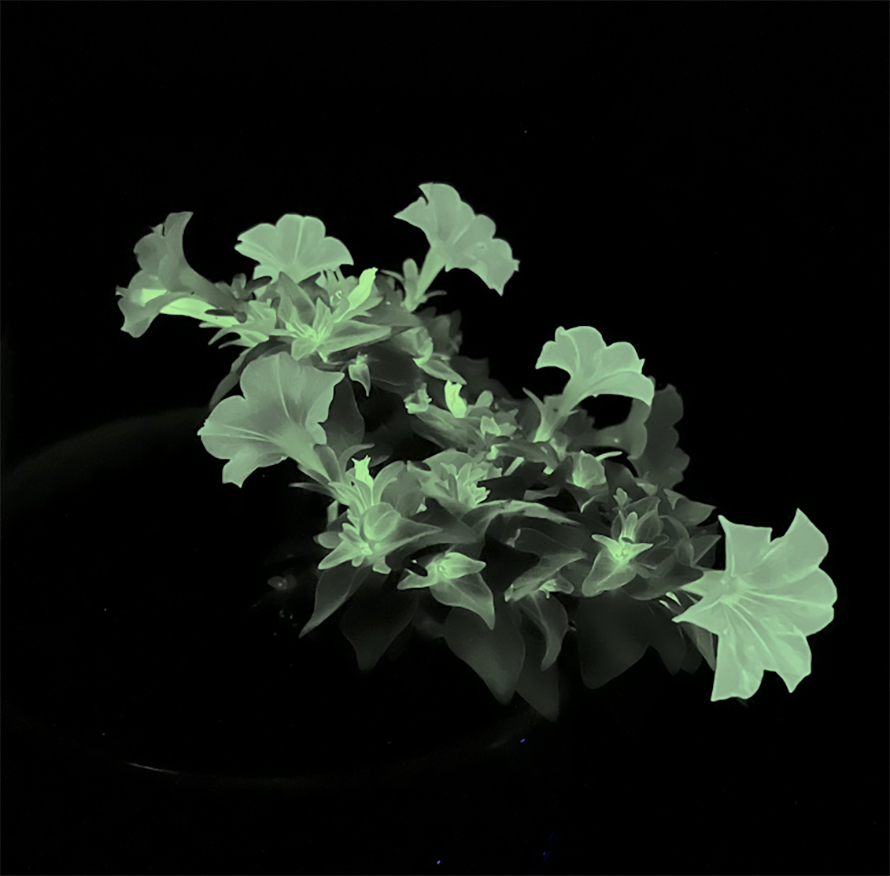
From glowing petunias to purple tomatoes
Today, DNA modification technologies are no longer confined to large-scale agribusiness – they are becoming available directly to consumers.
One approach is to mutate certain genes, like we did with our prickle-free plants. For example, scientists have created a mild-tasting but nutrient-dense mustard green by inactivating the genes responsible for bitterness. Silencing the genes that delay flowering in tomatoes has resulted in compact plants well suited to urban agriculture.
Another modification approach is to permanently transfer genes from one species to another, using recombinant DNA technology to yield what scientists call a transgenic organism.
At a recent party, I found myself crowded into a darkened bathroom to observe the faint glow of the host’s newly acquired firefly petunia, which contains the genes responsible for the ghost ear mushroom’s bioluminescent glow. Scientists have also modified a pothos houseplant with a gene from rabbits, which allows it to host air-filtering microbes that promote the breakdown of harmful volatile organic compounds, or VOCs.
Consumers can also grow the purple tomato, genetically engineered to contain pigment-producing genes from the snapdragon plant, resulting in antioxidant-rich tomatoes with a dark purple hue.
Risks and rewards
The introduction of genetically modified plants into the consumer market brings with it both exciting opportunities and potential challenges.
With genetically edited plants in the hands of the public, there could be less oversight over what people do with them. For instance, there is a risk of environmental release, which could have unforeseen ecological consequences. Additionally, as the market for these plants expands, the quality of products may become more variable, necessitating new or more vigilant consumer protection laws. Companies could also apply patent rules limiting seed reuse, echoing some of the issues seen in the agricultural sector.
The future of plant genetic technology is bright – in some cases, quite literally. Bioluminescent golf courses, houseplants that emit tailored fragrances or flowers capable of changing their color in response to spray-based treatments are all theoretical possibilities. But as with any powerful technology, careful regulation and oversight will be crucial to ensuring these innovations benefit consumers while minimizing potential risks.
This article is republished from The Conversation under a Creative Commons license. Read the original article.
![]()
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Building better tools to decipher the lipidome
Chemical engineer–turned–biophysicist Matthew Mitsche uses curiosity, coding and creativity to tackle lipid biology, uncovering PNPLA3’s role in fatty liver disease and advancing mass spectrometry tools for studying complex lipid systems.

Redefining lipid biology from droplets to ferroptosis
James Olzmann will receive the ASBMB Avanti Award in Lipids at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.