How DNA structure controls CRISPR
A team of scientists at Montana State University recently published new research that describes how DNA structure controls CRISPR, an immune system that helps bacteria defend themselves against viruses.

Andrew Santiago-Frangos is a postdoctoral fellow in the lab of professor Blake Wiedenheft in MSU’s Department of Microbiology and Cell Biology, which is housed in the College of Agriculture. Santiago-Frangos is the lead author on a new paper, which appeared in the journal Nature Structural and Molecular Biology, titled "Structure reveals why genome folding is necessary for site-specific integration of foreign DNA into CRISPR arrays” that explains how CRISPR systems record exposures to viral infections.
Santiago-Frangos joined the Wiedenheft Lab five years ago, drawn by its well-established program of ongoing CRISPR-related research.
“CRISPR has really blown up as a field since about 2012. I was excited to learn more about it,” he said. “Just like us, bacteria get sick from viral infections, and have evolved immune systems to fight those infections.”
CRISPRs are essential components of an adaptive immune system in bacteria that has recently gained considerable attention for its use in genome engineering. When bacteria are infected by a virus, some use CRISPR-based immune systems to snatch a piece of viral DNA and paste it into the bacteria’s genome.
“You can think of CRISPR as a molecular vaccination system that maintains a molecular record of past infections, and just like our own vaccinations, these systems help bacteria fight off reoccurring viral infections,” said Wiedenheft.
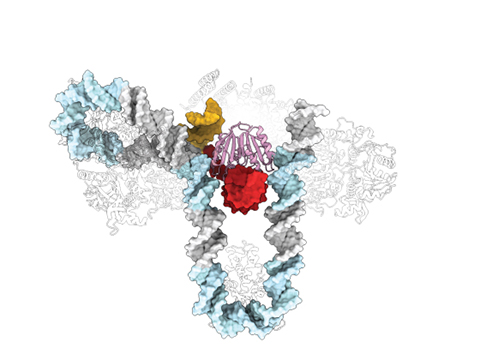
However, precisely how the bacteria snatch viral DNA and insert this DNA into the molecular vaccination system has remained unclear. To answer that question, the team used a new cyro-electron microscope in MSU’s Cryo-EM Facility to take photographs of the molecular machines caught in the act. Co-authors on the paper came from MSU’s Department of Microbiology and Cell Biology, the Department of Chemistry and Biochemistry in MSU’s College of Letters and Science, the New York Structural Biology Center and the Scripps Research Institute.
By shooting streams of electrons at the samples, Santiago-Frangos was able to determine the three-dimensional structure that explains how the machine bends and inserts DNA into the CRISPR.
“There was extra DNA flanking the CRISPR,” said Santiago-Frangos. “Previously, we hadn’t paid too much attention to that extra DNA, but now we’ve found strongly conserved sequences, which act like instructions for bending the DNA into a special shape. This is what helps the system identify where to insert foreign DNA.”
DNA is generally very rigid and should act like a steel rod at this scale, said Santiago-Frangos, so this degree of bending and folding was surprising.
“It’s important to understand why this happens because it gives us new knowledge of how this immune system works in a bacterial cell,” he said. “Now we’re exploring whether there are changes we can make to this a more efficient process.”
Art imitates science
Andrew Santiago–Frangos was one of the winners of the ASBMB’s inaugural Molecular Motifs bioart competition. His entry illustrated unique DNA bending. See all the winners, which will be printed in the ASBMB's calendar for 2024.
An image that Santiago-Frangos created of the unique DNA bending seen in this CRISPR complex (above) was selected by the American Society for Biochemistry and Molecular Biology in the organization’s Molecular Motifs bioart competition. The image will appear in the society's 2024 calendar.
Santiago-Frangos is continuing his research with the support of a prestigious MOSAIC award from the National Institutes of Health that he received last year. The award is designed to promote diversity in science and support early-career researchers in establishing their own labs. This semester, he will conclude his postdoctoral career at MSU and transition into leading his own lab at the University of Pennsylvania. He cited MSU’s faculty and administrative support and interdepartmental teamwork for aiding his success.
“I’m grateful for my time at Montana State University surrounded by mountains and great people,” said Santiago-Frangos. “I’ve learned a lot about grantsmanship and the creative side of science from my mentor, Dr. Wiedenheft. MSU fosters a welcoming and collaborative atmosphere, and I’ve greatly enjoyed working with research groups both in my own department of Microbiology and Cell Biology and in the Department of Chemistry and Biochemistry. I look forward to bringing MSU’s collaborative spirit with me as I transition to the next stage of my career as I open my own lab and join my new colleagues at the University of Pennsylvania.”
This article was first published by Montana State University. Read the original.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

