Plasma membrane is no barrier to free fatty acid
Our understanding of how long-chain fatty acids cross membranes is changing based on recent work by James Hamilton’s laboratory at Boston University and others. Their research shows that unlike nutrients such as glucose or amino acids, which require a transporter, fatty acids can diffuse spontaneously through protein-free lipid bilayers and cells’ plasma membranes.
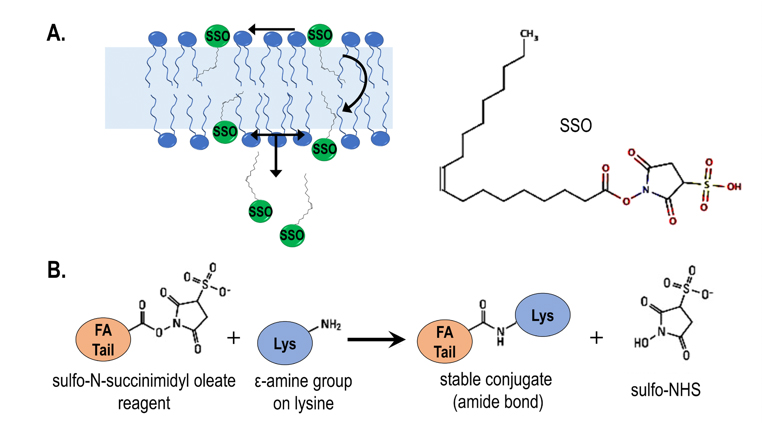
A recent paper in the Journal of Lipid Research by Anthony Jay and colleagues reveals how molecules previously thought to inhibit fatty acid transport specifically, including sulfosuccinimidyl oleate, or SSO, fit the diffusion model.
Control of fatty acid entry into cells is difficult to visualize. Researchers often have used fatty acid metabolites that become trapped in the cell to infer transport by plasma membrane proteins. The Hamilton lab has focused on distinguishing transmembrane movement from metabolism.
The lab made a seminal discovery that transport of fatty acids across a bilayer can be followed by pH changes. They combined a fluorescent fatty acid reporter with phospholipid vesicles enclosing a pH-probe to measure absorption in real time. They showed that natural fatty acids can acquire a proton near the outer leaflet, leading to equilibrium between neutral and ionized forms. The net neutral fatty acid spontaneously diffuses across the bilayer and then releases the proton near the inner leaflet, reducing the internal pH. This energy-free diffusion has been termed the “flip-flop” mechanism.
“Our studies also showed that all fatty acids studied … exhibit rapid flip-flop, and that this mechanism is reversible,” Hamilton said.
The researchers were excited by this finding but acknowledge that it does not exclude a role for proteins. Jay said, “If fatty acids can simply diffuse into cells, how could there be inhibitors of this transmembrane movement?”
The recent paper tests several proposed transport inhibitors, including the gold standard, SSO. Scientists had described SSO as a specific inhibitor of fatty acid entry into cells that acts on the fatty acid transporter CD36 without penetrating membranes. However, Hamilton’s team showed that SSO crosses membranes. Immunofluorescence using novel antibodies that bind SSO-linked fatty acids suggested that SSO modifies numerous proteins on the cell surface and interior, refuting the assumption of CD36 specificity.
The research informs nutritional considerations, Hamilton said. “Metabolism, and not the plasma membrane, controls the retention of fatty acids in cells, by trapping the fatty acids. Although membrane proteins may bind fatty acids or participate in metabolism, they cannot block diffusion in the surrounding bilayer.”
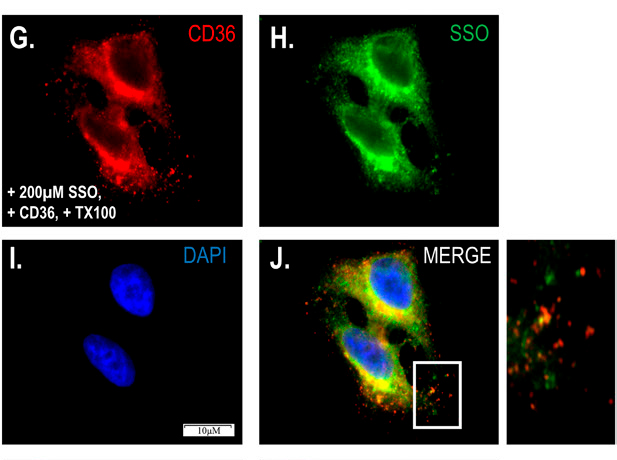
and the fatty acid transport inhibitor sulfosuccinimidyl oleate. The insert on the right suggest that SSO staining is not
exclusive to CD36.
In fact, cells with and without CD36 exhibited the same diffusion, but CD36 increased the content of intercellular lipids.
For nutrition, Hamilton emphasized, “Healthy fatty acids such as omega 3 fatty acids need to enter cells readily, whereas unhealthy fatty acids, such as trans fatty acids, cannot be excluded from cells and need to be reduced in the diet.”
Hamilton’s lab is moving on to clinical trials using a high concentration of beneficial fatty acids to improve stroke and heart attack outcomes. Meanwhile, he recommends taking omega-3 supplements and eating more nuts.
Another interpretation
According to Maastricht University researchers Jan Glatz and Joost Luiken, the Hamilton lab’s results also could be interpreted as a step toward integration of the argument that fatty acids diffuse across the cell membrane and the view that the protein CD36 is required for fatty acid uptake. In a Letter to the Editors of the JLR, Glatz and Luiken lay out their view that CD36 may be helpful but not necessary for lipid uptake.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

