AI unlocks the hidden grammar of gene regulation
The fruit fly Drosophila genome is like a 180-million letter book. Julia Zeitlinger, a professor at the Stowers Institute for Medical Research, is determined to read and dissect that book to understand how the sentences are built, and which words truly matter.
Zeitlinger studies how protein transcription factors, or TFs, bind to DNA to regulate gene expression, using fruit flies as her model. She likened TF binding sites, called motifs, to words in the genome’s book. But, unlike words, motifs have no fixed location, precise definition or predictable arrangement. Their meaning depends on position and context. To decode them, Zeitlinger needed more than biology. She found her solution in what was at the time an emerging and unfamiliar field — artificial intelligence.
Teaching AI to read the genome

An early adopter of using artificial intelligence, or AI, Zeitlinger began using it as an expert genome reader, allowing her to better define the linguistics of gene expression.
That innovative approach drew in bioinformatician Melanie Weilert, who, in 2017, was preparing to leave academia for industry because she struggled to connect with her work — until she joined the Zeitlinger lab.
“A lot of labs would pick a model system and work their way down to specific genes until they got to the crux of how a DNA sequence encodes what they’re interested in,” said Weilert, now a bioinformatician in Zeitlinger’s lab. “The Zeitlinger lab works in the opposite direction. The goal is to take well-studied examples and discover the general rules that apply to the entire genome.”
With 180 million letters to sift through, Zeitlinger needed tools to pinpoint which DNA base pairs might serve as TF binding sites. So, her lab developed chromatin immunoprecipitation-nexus, or ChIP-nexus, a method that maps TF genome binding sites down to the specific nucleotides.
This high-resolution method brought the entire genome under a magnifying glass. But, according to Zeitlinger, interpreting the data and rules of TF binding was daunting.
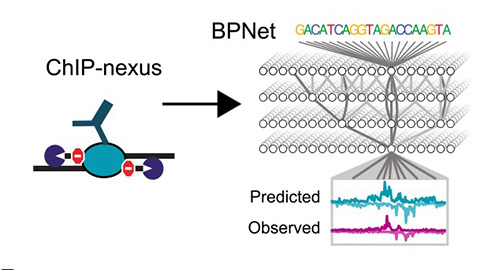
“We had this complex (ChIP-nexus) data we were trying to analyze,” Zeitlinger said. “We sort of knew what TF binding trends we should find, but finding general rules that predict binding genome-wide was impossible. It’s just too complex to dissect manually.”
That changed in 2015, when Zeitlinger heard Stanford University’s Anshul Kundaje describe how neural networks — machine learning models that can recognize, classify and predict patterns — could help unravel biological complexity.
Intrigued, Zeitlinger shared her ChIP-nexus data with Kundaje. One of his students, Žiga Avsec, now a research scientist at Google DeepMind, took on the challenge of making sense of the large datasets. His work gave rise to BPNet.

BPNet is a neural network that discovers genome-wide links between DNA sequence patterns and TF binding profiles from sequence alone, acting like an advanced grammar-decoder across the entire genome. Avsec called it a “specialized” AI tool that can predict experimental outcomes in seconds and at an incredible scale.
“Specialist AI tools excel at tasks that humans are not good at — they can find relationships between different pieces of experimental data and can computationally predict the experimental outcomes in seconds and at an incredible scale,” he said. “This allows scientists to improve their intuition about how nature works and allows them to narrow down the hypotheses to test in the lab.”
Zeitlinger said the precision and time savings from these tools are shaping both her research and the broader field, pushing science into new realms.
“We are going to get into a virtual biology world where we get knowledge at our fingertips and predict things really quickly,” Zeitlinger said. “And that’s super exciting,”
What AI revealed about chromatin and beyond
Zeitlinger quickly put BPNet to work. In a recent study, her team used it to predict how TFs interact while bound to DNA. Combining AI predictions and wet lab experiments, they showed that the program could forecast how one TF’s binding influences another, offering a new view of the genome’s syntax, and how gene expression is controlled.
BPNet’s abilities weren’t limited to TFs. Soon, the team applied it to nucleosomes, structures of DNA wrapped around a protein core. These structures not only package DNA but also affect gene regulation by controlling which sequences are “wound” and which are accessible.
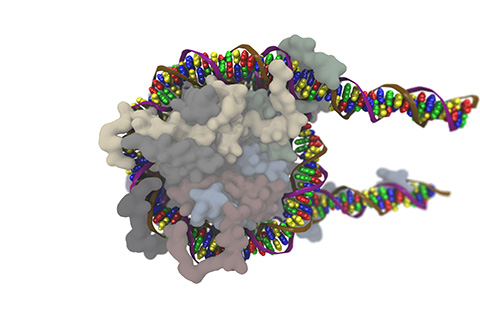
“They're not just packaging units, they have their own life,” Zeitlinger said. “Understanding what’s encoded in the nucleosome is not something we could easily address before, but with the (AI models) we can see things now that we were missing.”
In one study, Weilert, a bioinformatician in Zeitlinger’s lab, and colleagues combined experimental genomics with deep learning models to see how TFs unwind DNA during Drosophila embryo development. They identified several “sequence rules” that govern DNA accessibility and expression, better defining the multilayer coordination that influences development.
Weilert said AI has sped up discovery drastically. A decade ago, a Ph.D. student might spend years studying one motif. Now, a single experiment can reveal global patterns.
“It's not that they are reinventing science, but these tools provide us with the computational power to look at already existing data in completely different and powerful ways,” she said. “(These models) are just really effective pattern finders — they offer a high-dimensional capacity to identify biological phenomena that previously scientists would have to nail down using a specific hypothesis.”
Shaping how others use AI
Zeitlinger’s embrace of AI wasn’t always popular. Early on, some colleagues were skeptical. Over time, she adapted how she communicated its value and the way it intersected with her work, and the rise of general tools like ChatGPT made AI feel more familiar. Still, she remains cautious about its pitfalls.
“Part of me is concerned that the science will get so complex that it will be harder to distinguish good science from bad science,” she said. “It still feels a bit like the wild west.”
To help integrate AI into biological research, Zeitlinger makes sure that the conferences she organizes, including the American Society for Biochemistry and Molecular Biology’s evolution and core processes in gene expression conference, have speakers that apply advanced AI tools to the field. She also enjoys helping other labs navigate AI and was appointed at her institute to lead the Stowers Office of Scientific Leadership AI Initiative, which provides guidance and infrastructure support to researchers adopting AI tools in their work.
“I see it as a responsibility to make sure our institute maximally benefits from AI in a larger sense, while also avoiding its dangers,” Zeitlinger said. “My goal is to make sure that researchers are prepared for our changing landscape and maximally benefit from the new tools that become available.”
Avsec also sees AI as being transformative for science in the years ahead.
“(AI) tools, both general and specialized, will allow scientists to tackle more ambitious research questions in the coming years and thereby accelerate science,” he said.
Zeitlinger expects AI to keep shaping her own work. While Drosophila remains her model, she is also eyeing the human genome and how it governs development and function.
With AI in the toolkit, Zeitlinger hopes to predict the language rules behind healthy gene expression in humans — and uncover what goes wrong in diseases, such as cancer, neurological disorders and diabetes.
“I am very excited about the innovation that will come with using AI in biological research,” Zeilinger said. “Biology is so complex, and these tools are exactly what we need to put the pieces together.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

