Researchers discover toxin that kills bacteria in unprecedented ways
McMaster researchers have discovered a previously unknown bacteria-killing toxin that could pave the way for a new generation of antibiotics.
The study, led by John Whitney at the Michael G. DeGroote Institute for Infectious Disease Research, shows that the bacterial pathogen Pseudomonas aeruginosa, known to cause hospital-acquired infections such as pneumonia, secretes a toxin that has evolved to kill other species of bacteria.

For Whitney, the key aspect of his discovery is not just that this toxin kills bacteria, but how it does so.
“This research is significant, because it shows that the toxin targets essential RNA molecules of other bacteria, effectively rendering them non-functional,” says Whitney, an associate professor in the department of biochemistry and biomedical sciences.
“Like humans, bacteria require properly functioning RNA in order to live.”
First study author Nathan Bullen, a graduate student in biochemistry and biomedical sciences, describes it as “a total assault on the cell” because of the number of essential pathways depend on functional RNAs.
Whitney and Bullen, together with colleagues at Imperial College London and the University of Manitoba, have studied this toxin for nearly three years to understand exactly how it functions at a molecular level.
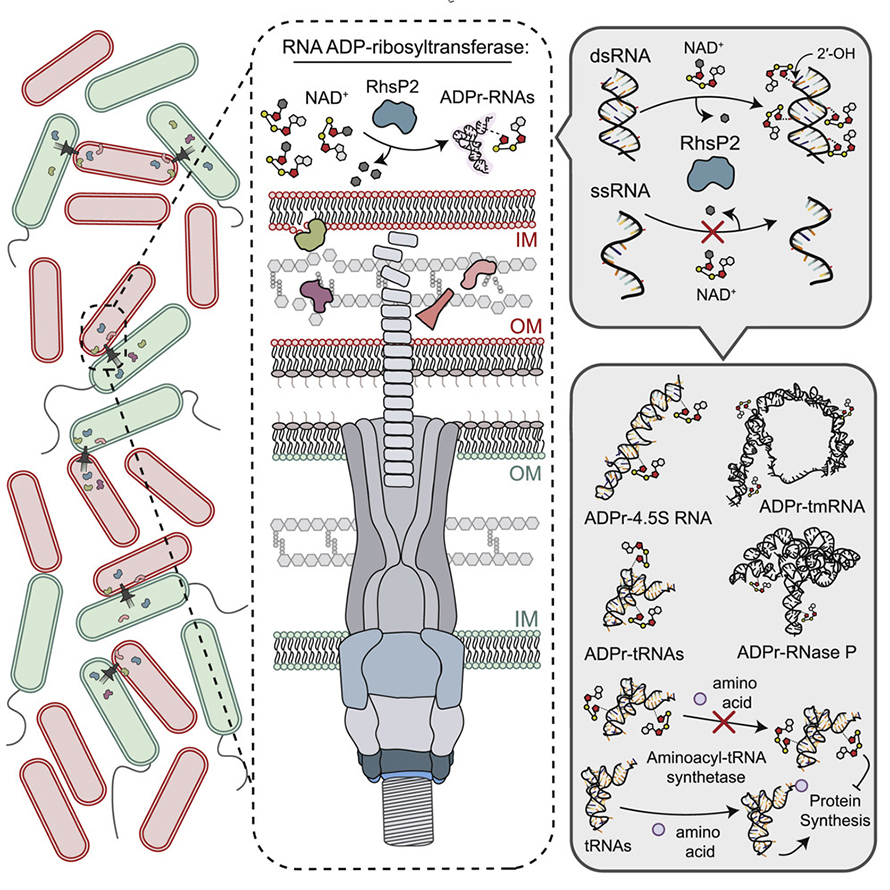
The breakthrough, published in the journal Molecular Cell, was achieved by Bullen after rigorous experimentation on common targets of toxins, such as protein and DNA molecules, before eventually testing the toxin against RNA.
This discovery breaks well-established precedents set by protein-targeting toxins secreted by other bacteria, such as those that cause cholera and diphtheria.
Researchers say that this development holds great potential for future research that could eventually lead to new innovations that combat infection-causing bacteria.
Whitney says future antibiotic development can build on the newly discovered vulnerability.
This article was republished with permission from the Institute for Infectious Disease Research at McMaster University. Read the original.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

