Is location everything?
Cholesterol is an essential structural component of cell membranes, where it also can function as a regulator of cellular processes.
Researchers have long known that cholesterol regulates the structure and function of integral proteins in the plasma membrane, such as ion channels and G protein–coupled receptors. Evidence also indicates that cholesterol in the inner leaflet of the plasma membrane regulates cellular signaling by binding to signaling proteins. However, no techniques exist that allow site-specific control of cholesterol levels.
In a recent study in the Journal of Lipid Research, Wonhwa Cho and his team at the University of Illinois Chicago developed a technique to precisely control cholesterol levels. Cho believes the new method will benefit biomedical research that can help improve the treatment of cholesterol-associated diseases.
“Many years ago, we discovered and published that cholesterol in the inner leaflet of the plasma membrane can activate cellular processes leading to cell overgrowth,” Cho said. “Others have also reported site-specific actions of cholesterol in the cell. We thus needed a tool that can help us unambiguously elucidate the site-specific function of cholesterol.”
Although researchers can use standard methods such as chemical extraction and enrichment of cholesterol by methyl-beta-cyclodextrin and inhibition of new cellular cholesterol biosynthesis by statins, these techniques do not allow the spatiotemporal depletion of cholesterol, and they can be toxic to the cells.
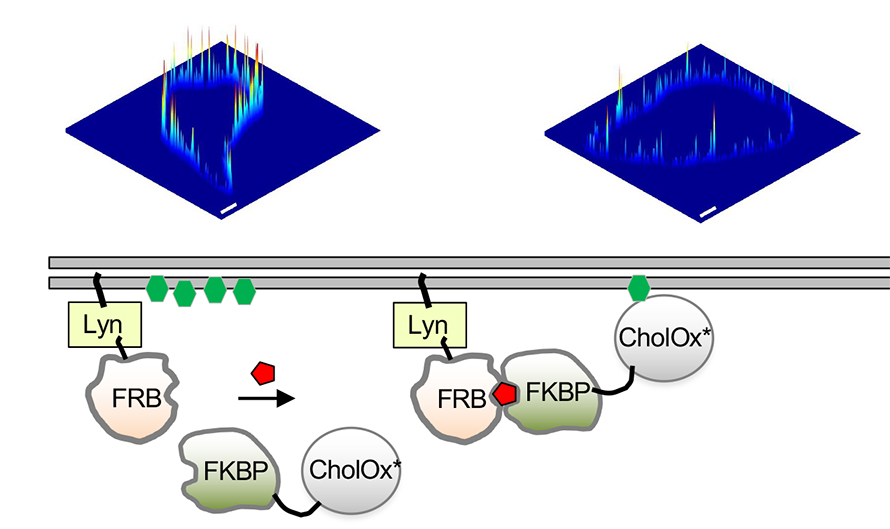
Cho’s method takes advantage of an inducible protein dimerization system and the cholesterol-depleting abilities of Streptomyces species cholesterol oxidase, an enzyme that catalyzes the breakdown of cholesterol in a two-step process. First, it must bind to the membrane; then cholesterol is converted to cholest-4-en-3-one, or cholestenone.
The researchers rationally designed a mutant of cholesterol oxidase, referred to as WVR, which displayed compromised membrane binding with no significant change in the catalytic function of cholesterol oxidase. Cho’s team used the WVR mutant to design a system that artificially targets cholesterol oxidase to the plasma membrane as a spatiotemporally inducible cholesterol depletion agent.
Researchers often use protein dimer formation in response to external stimulation to understand the role of protein–protein interactions in cellular functions. The FRB–FKBP dimerization system uses rapamycin, an antifungal antibiotic that simultaneously can bind the FK506 binding protein and the FKBP–rapamycin binding domain of the mammalian target of rapamycin, known respectively as the FKBP and the FRB, resulting in heterodimer formation. Proteins of interest can be fused to FKBP or FRB, and dimerization can be induced by adding rapamycin or analogs such as rapalog.
Cho’s team conjugated the mutant cholesterol oxidase to FKBP. They docked the FRB domain to the membranes using a short peptide sequence, Lyn, that targets proteins to the plasma membrane. They achieved site-specific targeting of cholesterol oxidase by adding rapalog, which induced the dimerization of FKBP–FRB and translocation of the mutant cholesterol oxidase to the plasma membrane. Site-specific depletion of cholesterol allowed Cho’s team to determine unambiguously the different functions of cholesterol in the cytosolic leaflets of the plasma membrane and lysosomes.
“Cholesterol has been linked to various cancers, including breast and colorectal cancers,” Cho said. “Our new tools will be very valuable in elucidating the mechanistic link between cellular cholesterol levels and oncogenic cellular process. This in turn will aid in development of new cancer drugs that modulate cholesterol-mediated oncogenic cellular processes.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Building better tools to decipher the lipidome
Chemical engineer–turned–biophysicist Matthew Mitsche uses curiosity, coding and creativity to tackle lipid biology, uncovering PNPLA3’s role in fatty liver disease and advancing mass spectrometry tools for studying complex lipid systems.

Redefining lipid biology from droplets to ferroptosis
James Olzmann will receive the ASBMB Avanti Award in Lipids at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Women’s health cannot leave rare diseases behind
A physician living with lymphangioleiomyomatosis and a basic scientist explain why patient-driven, trial-ready research is essential to turning momentum into meaningful progress.

Life in four dimensions: When biology outpaces the brain
Nobel laureate Eric Betzig will discuss his research on information transfer in biology from proteins to organisms at the 2026 ASBMB Annual Meeting.

