Is location everything?
Cholesterol is an essential structural component of cell membranes, where it also can function as a regulator of cellular processes.
Researchers have long known that cholesterol regulates the structure and function of integral proteins in the plasma membrane, such as ion channels and G protein–coupled receptors. Evidence also indicates that cholesterol in the inner leaflet of the plasma membrane regulates cellular signaling by binding to signaling proteins. However, no techniques exist that allow site-specific control of cholesterol levels.
In a recent study in the Journal of Lipid Research, Wonhwa Cho and his team at the University of Illinois Chicago developed a technique to precisely control cholesterol levels. Cho believes the new method will benefit biomedical research that can help improve the treatment of cholesterol-associated diseases.
“Many years ago, we discovered and published that cholesterol in the inner leaflet of the plasma membrane can activate cellular processes leading to cell overgrowth,” Cho said. “Others have also reported site-specific actions of cholesterol in the cell. We thus needed a tool that can help us unambiguously elucidate the site-specific function of cholesterol.”
Although researchers can use standard methods such as chemical extraction and enrichment of cholesterol by methyl-beta-cyclodextrin and inhibition of new cellular cholesterol biosynthesis by statins, these techniques do not allow the spatiotemporal depletion of cholesterol, and they can be toxic to the cells.
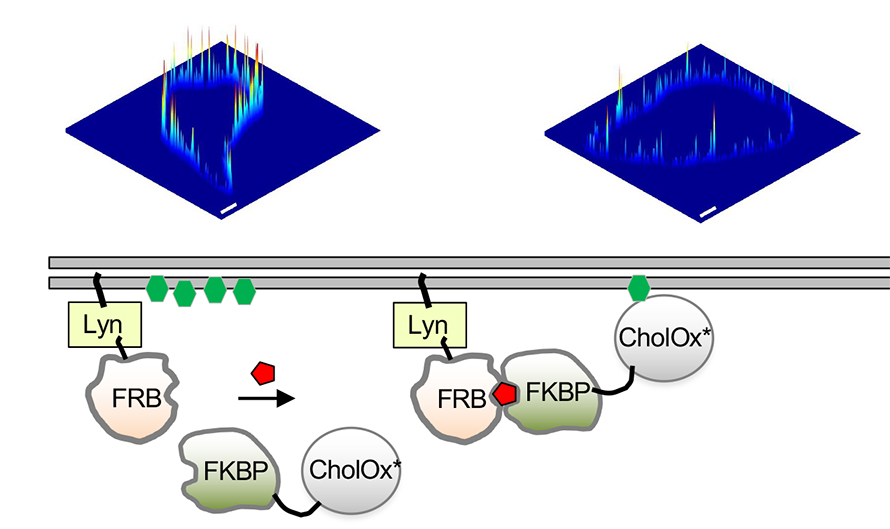
Cho’s method takes advantage of an inducible protein dimerization system and the cholesterol-depleting abilities of Streptomyces species cholesterol oxidase, an enzyme that catalyzes the breakdown of cholesterol in a two-step process. First, it must bind to the membrane; then cholesterol is converted to cholest-4-en-3-one, or cholestenone.
The researchers rationally designed a mutant of cholesterol oxidase, referred to as WVR, which displayed compromised membrane binding with no significant change in the catalytic function of cholesterol oxidase. Cho’s team used the WVR mutant to design a system that artificially targets cholesterol oxidase to the plasma membrane as a spatiotemporally inducible cholesterol depletion agent.
Researchers often use protein dimer formation in response to external stimulation to understand the role of protein–protein interactions in cellular functions. The FRB–FKBP dimerization system uses rapamycin, an antifungal antibiotic that simultaneously can bind the FK506 binding protein and the FKBP–rapamycin binding domain of the mammalian target of rapamycin, known respectively as the FKBP and the FRB, resulting in heterodimer formation. Proteins of interest can be fused to FKBP or FRB, and dimerization can be induced by adding rapamycin or analogs such as rapalog.
Cho’s team conjugated the mutant cholesterol oxidase to FKBP. They docked the FRB domain to the membranes using a short peptide sequence, Lyn, that targets proteins to the plasma membrane. They achieved site-specific targeting of cholesterol oxidase by adding rapalog, which induced the dimerization of FKBP–FRB and translocation of the mutant cholesterol oxidase to the plasma membrane. Site-specific depletion of cholesterol allowed Cho’s team to determine unambiguously the different functions of cholesterol in the cytosolic leaflets of the plasma membrane and lysosomes.
“Cholesterol has been linked to various cancers, including breast and colorectal cancers,” Cho said. “Our new tools will be very valuable in elucidating the mechanistic link between cellular cholesterol levels and oncogenic cellular process. This in turn will aid in development of new cancer drugs that modulate cholesterol-mediated oncogenic cellular processes.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

When oncogenes collide in brain development
Researchers at University Medical Center Hamburg, found that elevated oncoprotein levels within the Wnt pathway can disrupt the brain cell extracellular matrix, suggesting a new role for LIN28A in brain development.

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

