JBC: A shadowy organizational hub in cells
On a cellular level, we are all hanging on by delicate threads. All cells are crisscrossed by a network of strands called microtubules, which act as railroad tracks that move cargo around the cell, as winch cables that separate chromosomes during cell division and as scaffolding components that give a cell its shape.
Because of its essential role in the cell cycle, microtubule assembly is the target of essential anti-cancer chemotherapies (paclitaxel, for example), which stop out-of-control cell division by destabilizing microtubules. Now, researchers have shed light on the role that a large, enigmatic protein plays in assembling microtubules, paving the way for better treatments. The results of the research were published in the Journal of Biological Chemistry.
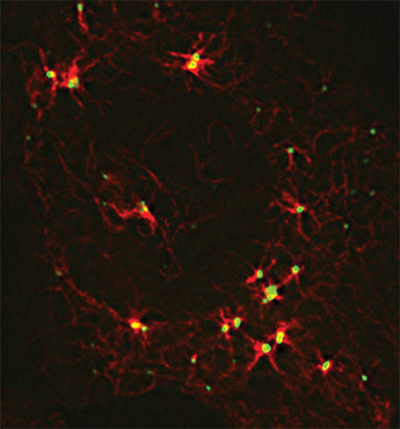 A large protein coordinates cellular components required for microtubule assembly.Courtesy of James Goldenring, Vanderbilt University
A large protein coordinates cellular components required for microtubule assembly.Courtesy of James Goldenring, Vanderbilt University
In 1999, James Goldenring’s research team at Vanderbilt University showed that protein kinase A-anchoring protein 350, or AKAP350, is a component of the centrosome, a center of microtubule organization in human cells. The team later showed that microtubules did not form efficiently without AKAP350. But the way in which AKAP350 regulated microtubule formation was difficult to understand, largely because of the technical challenges posed by AKAP350’s heft.
“Since this protein is so huge, it’s very difficult to study it,” said Elena Kolobova, the research scientist in Goldenring’s laboratory who led the new study. “A few years ago, we finally came to develop synthetic constructs of (AKAP350), which allowed us to go to the next level of evaluation and function.”
Using a combination of detailed biochemical analyses and super-resolution microscopy, the team finally was able to gain some understanding of the complex roles that AKAP350 plays in regulating microtubules in cells. AKAP350 formed a physical bridge spanning components of the centrosome. And AKAP350 appeared to recruit multiple proteins involved in building microtubules, coordinating their function in one spot.
“I like to call this thing Deep Space Nine. Everybody comes to hang out at AKAP350,” Goldenring said. “I think we’ve only scratched the surface of the structural organization that this protein is probably providing.”
Mutations in AKAP350 have been associated with cardiac arrhythmias, so it will be of interest to see whether the protein’s role in microtubule assembly contributes to proper heart function as well.
“I think (AKAP350) is a fundamental regulator of cell function,” Goldenring said. “So we need to know a lot more about this protein before we can even begin looking at what it might mean for disease.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

.jpg?lang=en-US&width=300&height=300&ext=.jpg)