New clues reveal how cells respond to stress
Inflammasome protein complexes form in response to signals associated with danger, such as an infection or environmental stress, and trigger the innate immune response. The serine protease dipeptidyl peptidase 9, or DPP9, forms a dimer in its active conformation and interacts with components of inflammasomes to prevent unnecessary activation. Scientists know that synthetic inhibitors of DPP9 activate certain inflammasomes. However, whether a cell-intrinsic molecule can inhibit this enzyme remains an open question. Therefore, Lydia Tsamouri, Jeffrey Hsiao and colleagues from the Weill Cornell Graduate School of Medical Sciences and Memorial Sloan Kettering Cancer Center investigated DPP9 interaction partners. They examined a connection between DPP9 and redox sensor KEAP1 in their recent Journal of Biological Chemistry article.
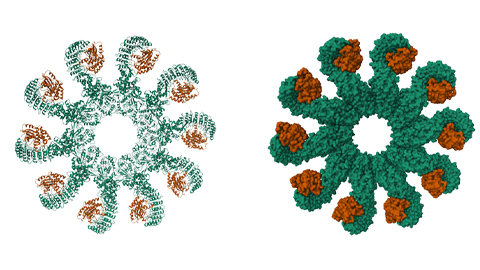
Using a fluorogenic probe that functions as a DPP9 substrate, the authors established that KEAP1 inhibits DPP9 activity in cells. They also found that KEAP1 can only inhibit DPP9 when both are introduced into cells at the same time via transfection with complementary DNA, or cDNA, that encodes each protein, before DPP9 dimerizes; newly introduced KEAP1 could not inhibit DPP9 already present in cells. The researchers hypothesized that KEAP1 interacts with DPP9 in a state different from its folded dimeric structure and that a cellular event or biomolecule could force DPP9 to adopt this alternative conformation. They tested multiple compounds, including electron transport chain inhibitors and oxidants like hydrogen peroxide, but they have not yet found a condition that leads to KEAP1–DPP9 complex formation and DPP9 inactivation.
Future experiments will focus on identifying a signal or molecules that could change DPP9’s conformation and whether the DPP9–KEAP1 interaction directly initiates inflammasome activation. Outlining the full DPP9 pathway involving inflammasomes will help scientists understand how cells convert danger signals into immune action and restrain unnecessary activation.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

