Engineering cells to broadcast their behavior can help scientists study their inner workings
Waves are ubiquitous in nature and technology. Whether it’s the rise and fall of ocean tides or the swinging of a clock’s pendulum, the predictable rhythms of waves create a signal that is easy to track and distinguish from other types of signals.
Electronic devices use radio waves to send and receive data, like your laptop and Wi-Fi router or cellphone and cell tower. Similarly, scientists can use a different type of wave to transmit a different type of data: signals from the invisible processes and dynamics underlying how cells make decisions.
I am a synthetic biologist, and my research group developed a technology that sends a wave of engineered proteins traveling through a human cell to provide a window into the hidden activities that power cells when they’re healthy and harm cells when they go haywire.
Waves are a powerful engineering tool
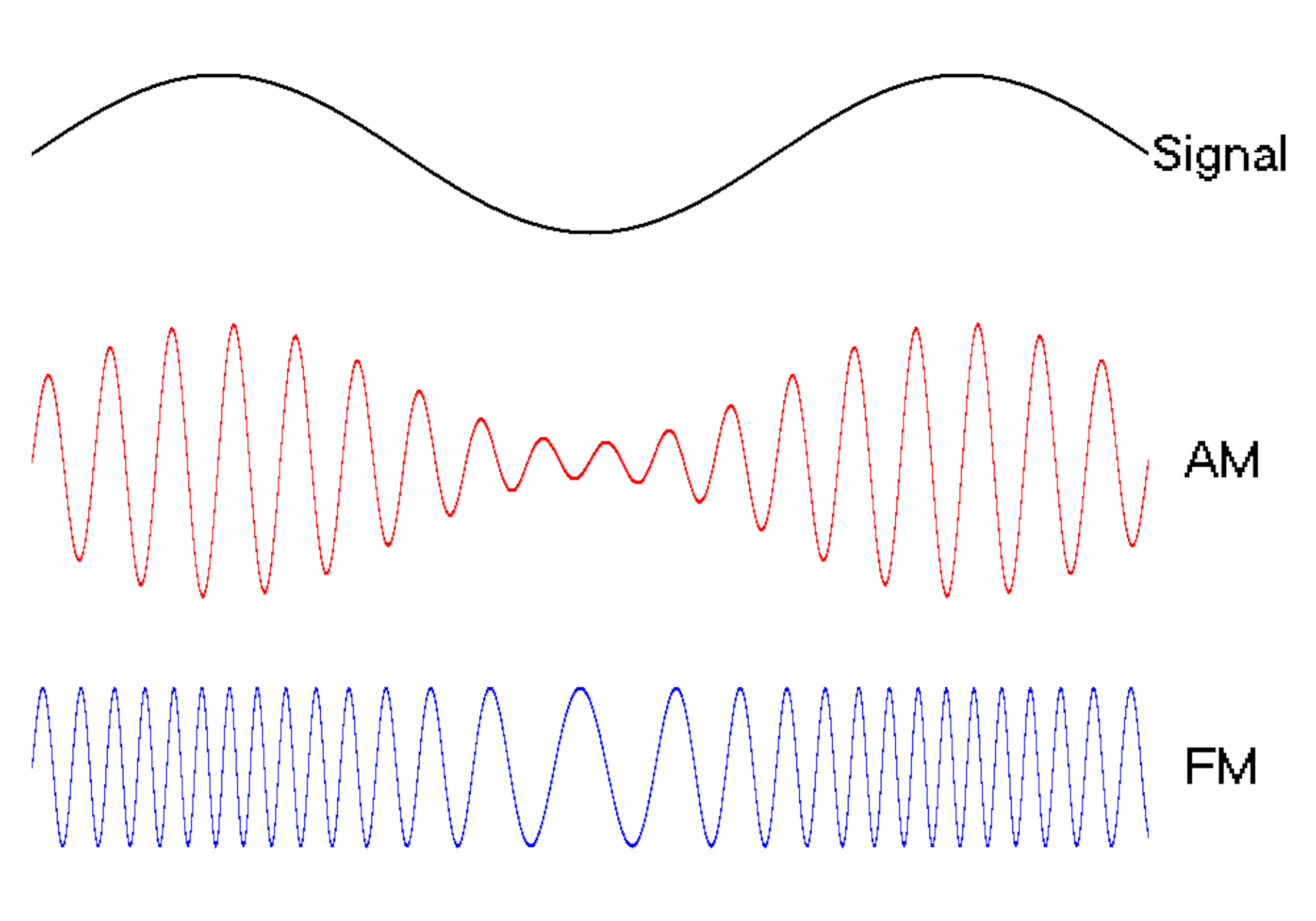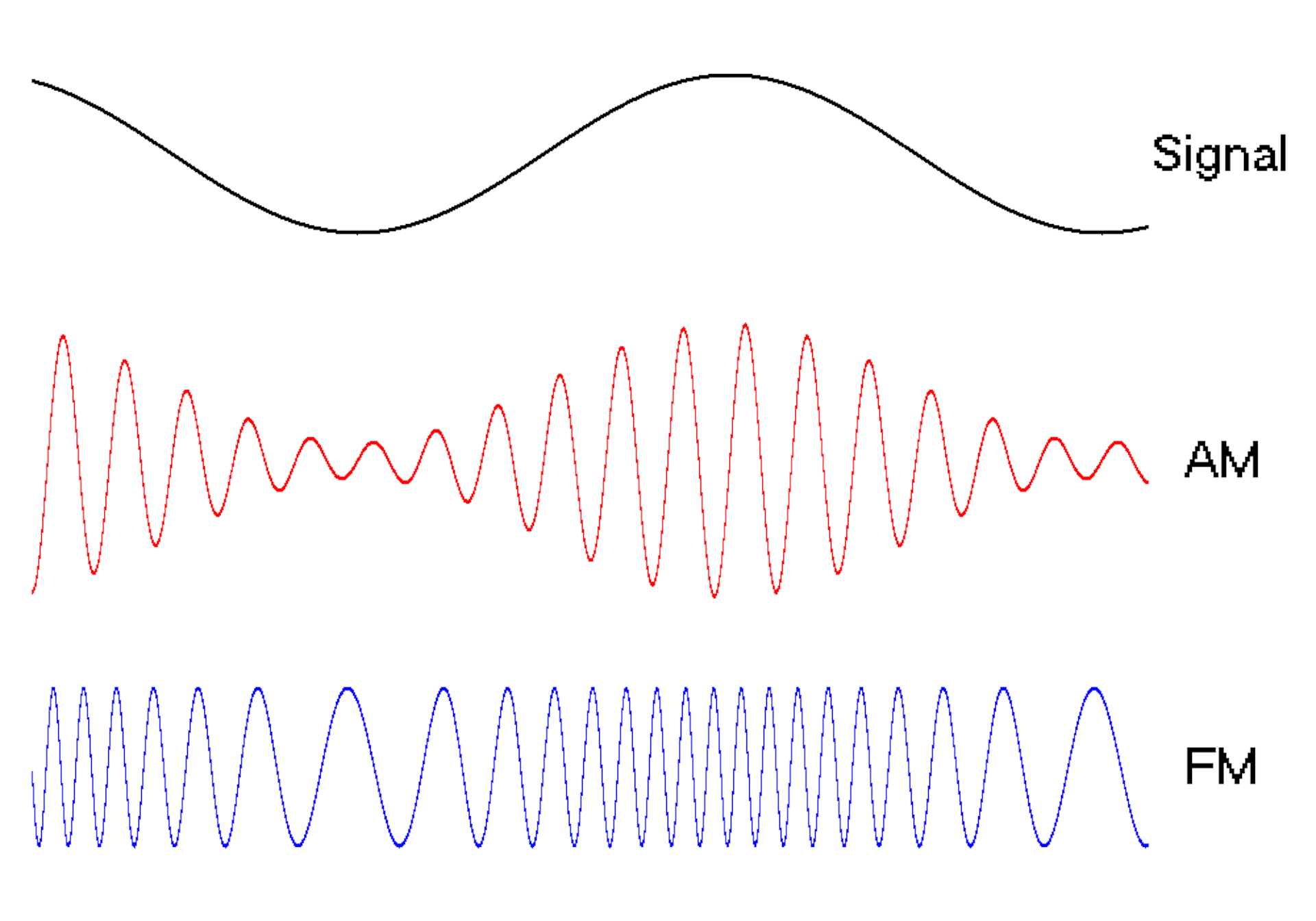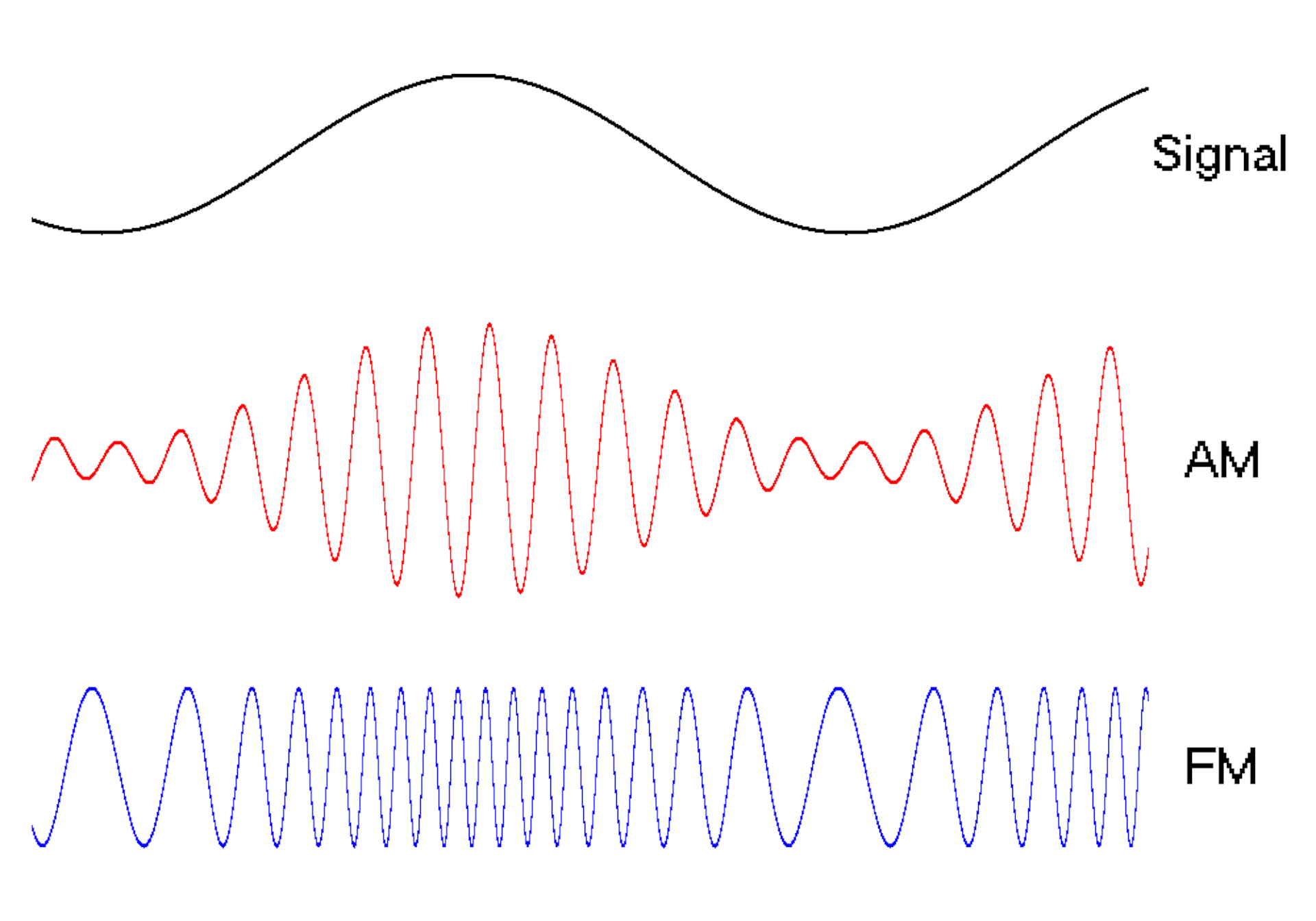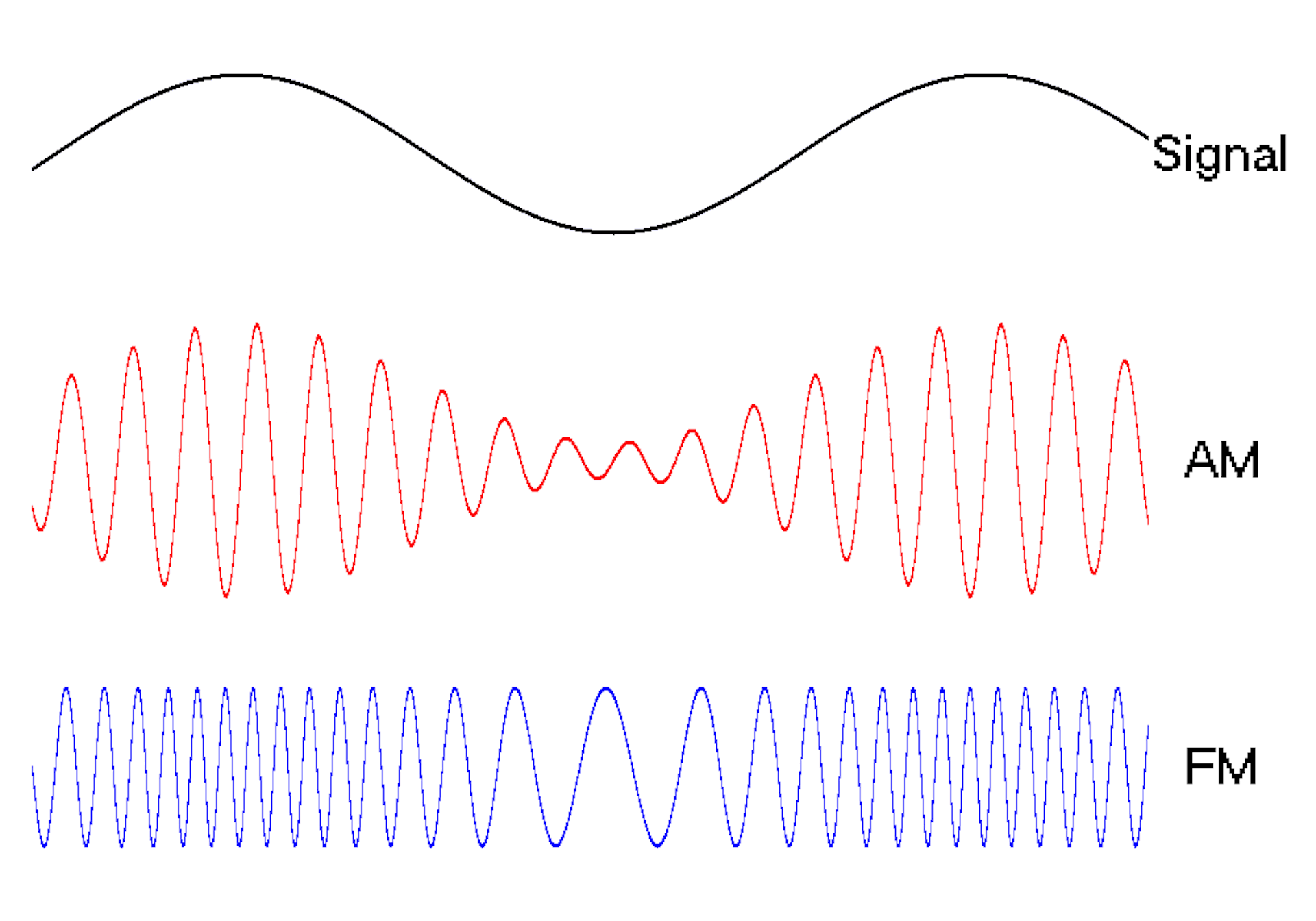
The oscillating behavior of waves is one reason they’re powerful patterns in engineering.
For example, controlled and predictable changes to wave oscillations can be used to encode data, such as voice or video information. In the case of radio, each station is assigned a unique electromagnetic wave that oscillates at its own frequency. These are the numbers you see on the radio dial.
Scientists can extend this strategy to living cells. My team used waves of proteins to turn a cell into a microscopic radio station, broadcasting data about its activity in real time to study its behavior.

Turning cells into radio stations
Studying the inside of cells requires a kind of wave that can specifically connect to and interact with the machinery and components of a cell.
While electronic devices are built from wires and transistors, cells are built from and controlled by a diverse collection of chemical building blocks called proteins. Proteins perform an array of functions within the cell, from extracting energy from sugar to deciding whether the cell should grow.
Protein waves are generally rare in nature, but some bacteria naturally generate waves of two proteins called MinD and MinE – typically referred to together as MinDE – to help them divide. My team discovered that putting MinDE into human cells causes the proteins to reorganize themselves into a stunning array of waves and patterns.
On their own, MinDE protein waves do not interact with other proteins in human cells. However, we found that MinDE could be readily engineered to react to the activity of specific human proteins responsible for making decisions about whether to grow, send signals to neighboring cells, move around and divide.
The protein dynamics driving these cellular functions are typically difficult to detect and study in living cells because the activity of proteins is generally invisible to even high-power microscopes. The disruption of these protein patterns is at the core of many cancers and developmental disorders.
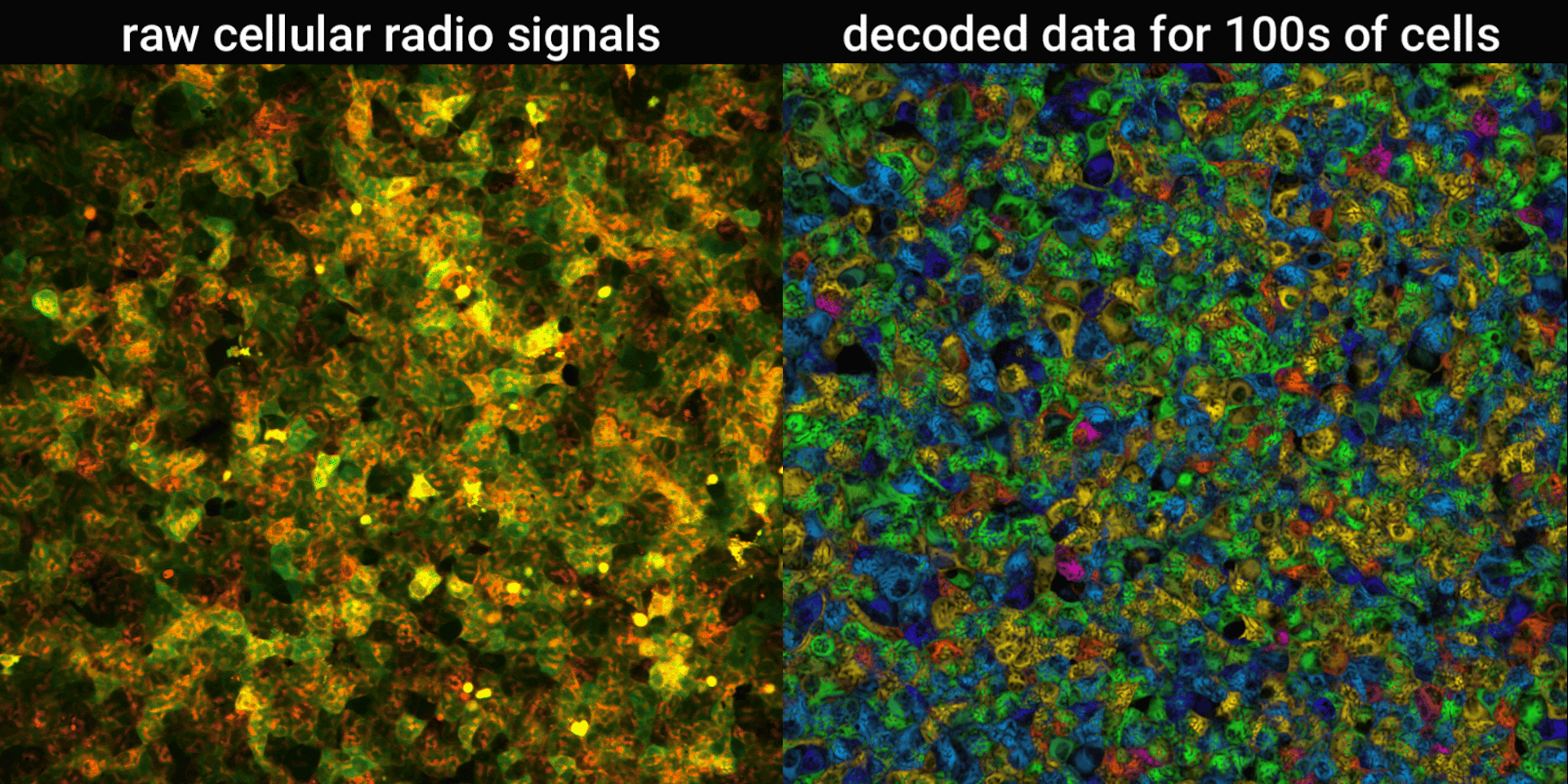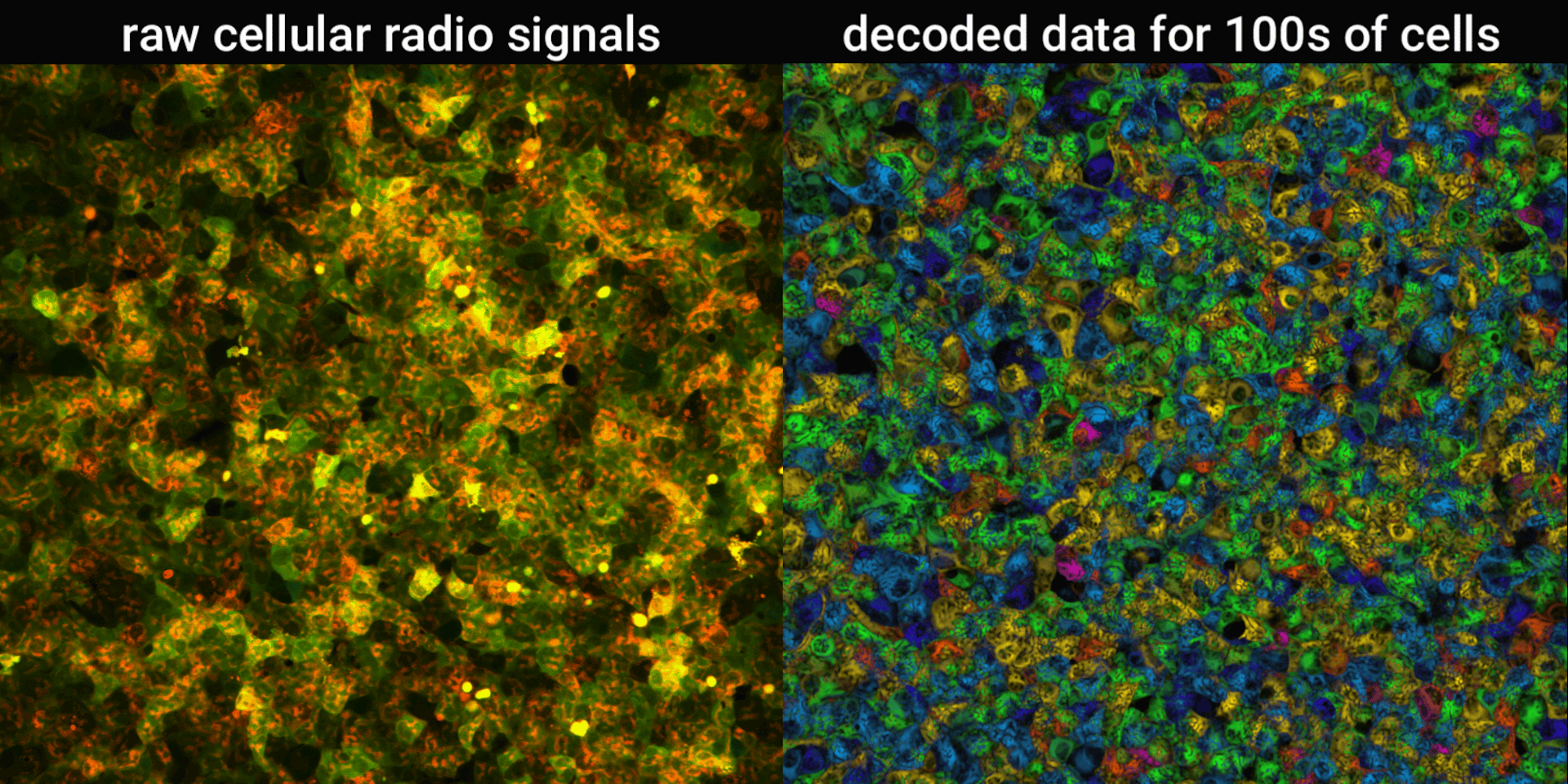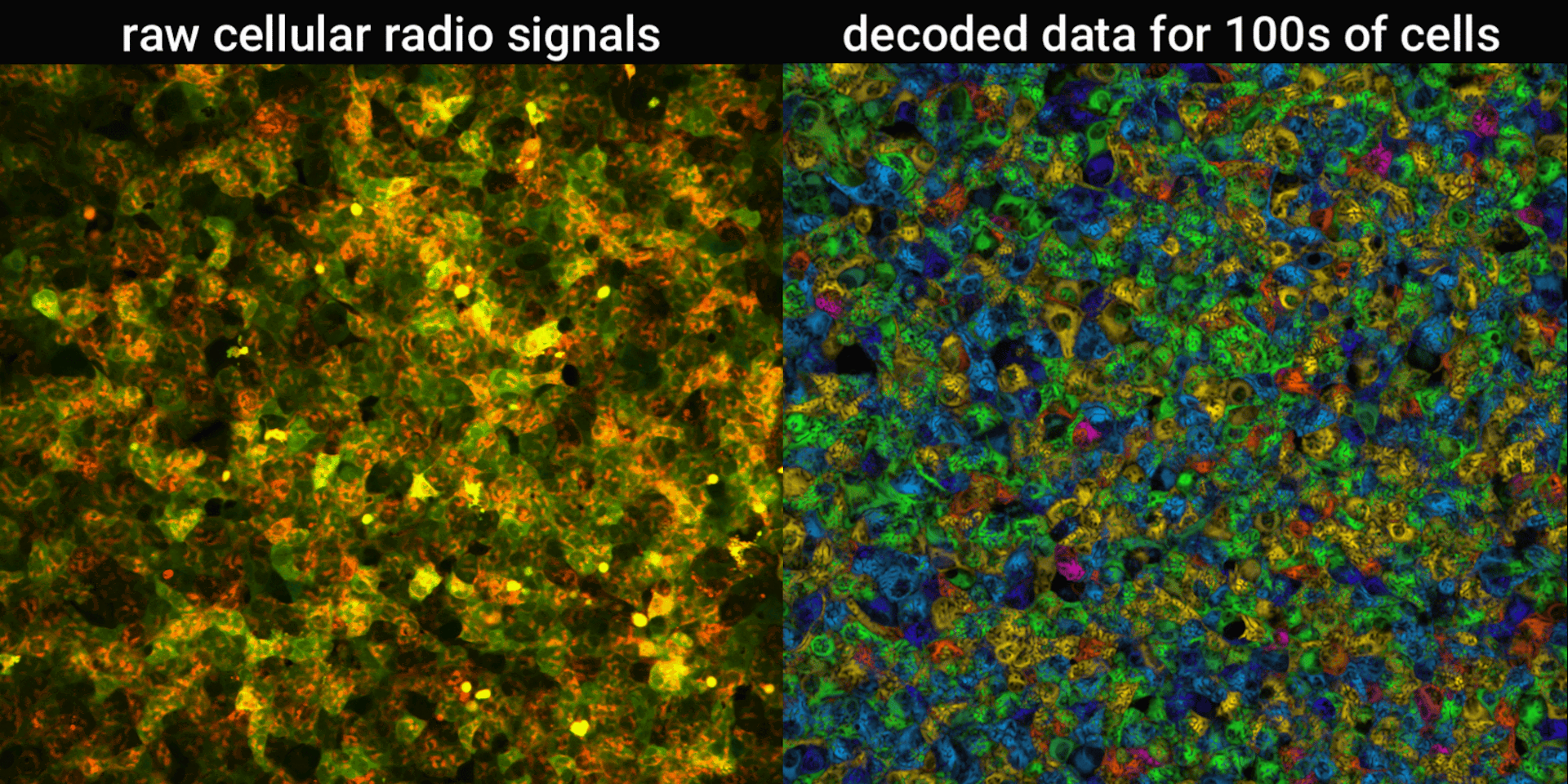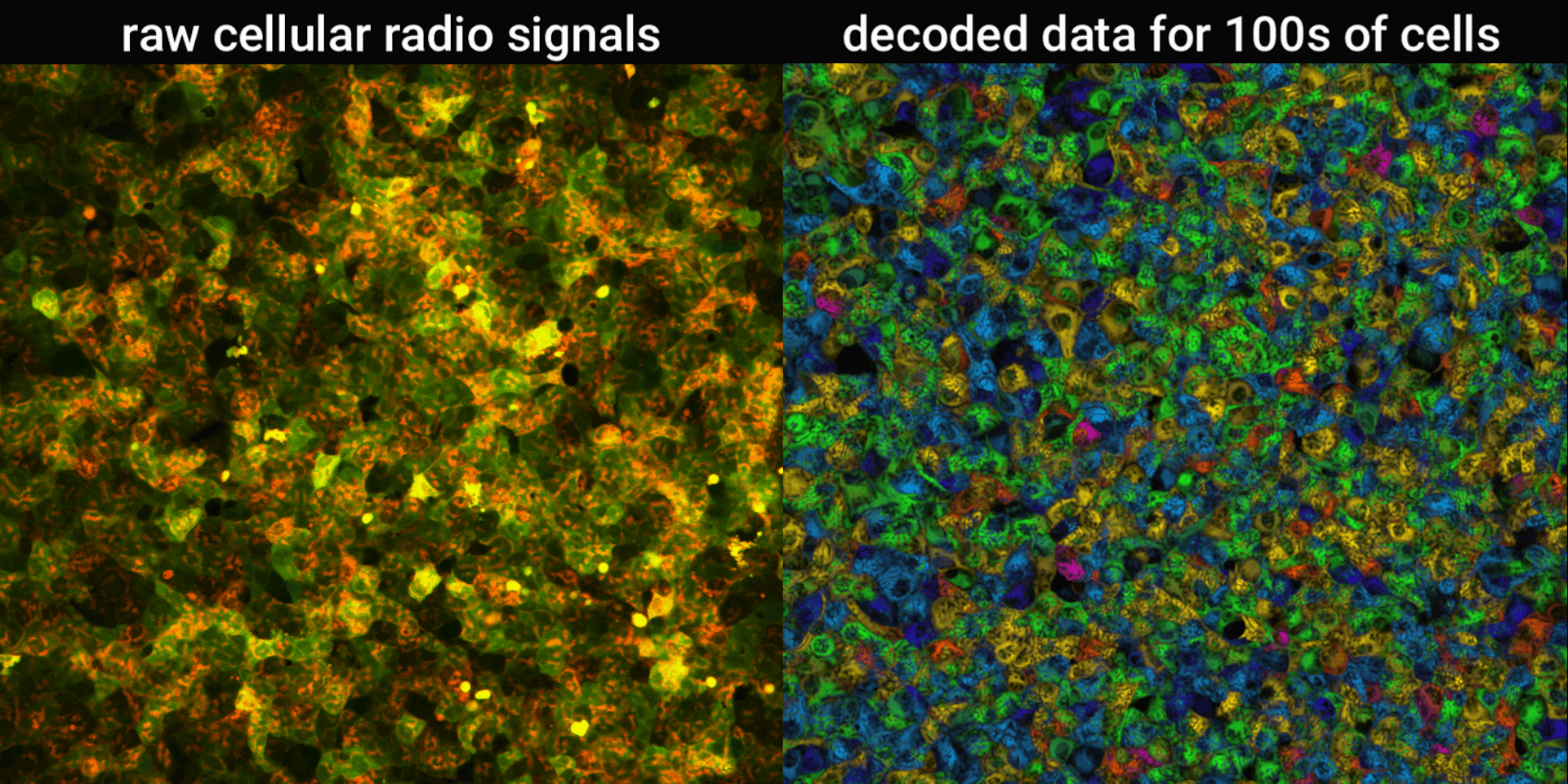
We engineered connections between MinDE protein waves and the activity of proteins responsible for key cellular processes. Now, the activity of these proteins trigger changes in the frequency or amplitude of the protein wave, just like an AM/FM radio. Using microscopes, we can detect and record the unique signals individual cells are broadcasting and then decode them to recover the dynamics of these cellular processes.
We have only begun to scratch the surface of how scientists can use protein waves to study cells. If the history of waves in technology is any indicator, their potential is vast.
This article is republished from The Conversation under a Creative Commons license. Read the original article.
![]()
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

