Examining mechanisms of protein complex at a basic cell biological level
A new study highlighting the importance of a large protein complex called the exocyst in cell growth, division and communication reveals new functions and mechanisms that are essential to how molecules move across a membrane through vesicles in a cell.

Understanding how these mechanisms work in normal cells at the basic biological level will inform future research into how those functions are disrupted in developmental and neurological disorders.
“It’s the first time a role for membrane fusion has been described for this complex and it’s a breakthrough in how we think about the way the exocyst complex works,” said Mary Munson, professor and vice chair for diversity in the Department of Biochemistry & Molecular Biotechnology, associate vice provost for equity in science in the Office of Health Equity and a co-corresponding author on the study published in Nature Structural & Molecular Biology. The research was done in collaboration with Tae-Young Yoon, professor of biological sciences at the School of Biological Sciences and Institute for Molecular Biology and Genetics at Seoul National University in Seoul, South Korea.
“Until our collaborative study, exocyst was understood to recognize and possibly tether secretory vesicles to the cell membrane, prior to exocytosis. In this study, we reveal biophysical studies that indicate exocyst playing several critical roles that directly facilitate fusion of the vesicles with the cell membrane to drive cargo delivery,” Munson said.
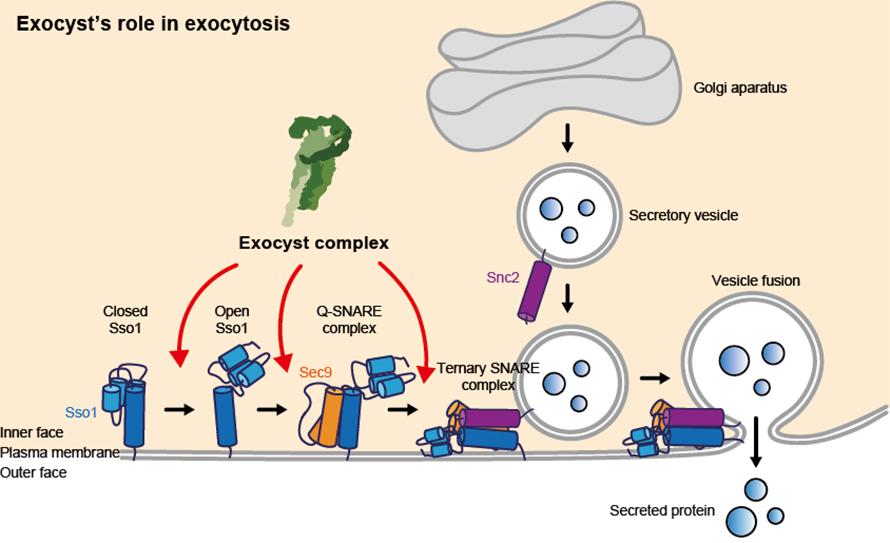
Robust control of exocytosis is critical for all cells to grow, divide and communicate properly. Dysfunction of exocyst regulation has been linked to many physiological problems in different organisms.
“It’s really an exciting development in our field, as the exocyst complex had not previously been shown to directly control vesicle fusion,” Munson said. “The complex recognizes the right vessel, the right place on the cell surface and the right time for the cargo to be delivered. It does that by talking to the membrane fusion protein.”
In 2023, Munson received a National Institutes of Health award for $3.3 million over five years to support this research.
Munson was recently named a 2024 recipient of a Zenith Award from the Association for Women in Science. The award honors senior career professionals with a lifetime of innovative achievements in STEM and a commitment to workplace diversity.
This article is republished from the UMass Chan Medical School News. Read the original here.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

