
The gut-brain connection
Benjamin Stecher was living in Shanghai when he first noticed the tremor. He had moved to China not long after college and landed a job teaching English at a company called San Li Around America. Within a couple of years, he was a managing partner. Meanwhile, the rigidity and shaking in his right foot and hand slowly got worse.

During a trip home to Canada in 2013, he saw a movement-disorder specialist who quickly diagnosed the problem as a symptom of early-onset Parkinson’s disease. Stecher was just 29 years old.
Experts estimate that by the time a Parkinson’s diagnosis is made, more than half of the dopaminergic neurons in a section of the brain controlling movement already have died. Aggregates of the protein alpha-synuclein form clumps known as Lewy bodies that disrupt neuronal function and eventually kill the cells. While some treatments, such as the drug levodopa, control patients’ tremors, there are currently no methods to block the ongoing damage (see: The shaking palsy). As neurodegeneration spreads, patients suffer a gradual loss of movement and independence.
“It’s not easy figuring out how to move on with life as a young man faced with that kind of fate,” Stecher said.
He tried to work around his symptoms, but they continued to worsen. At age 32, he moved home to Toronto to focus on his health.
Stecher asked his doctor a lot of questions. Then he started asking other experts. Soon, he found himself immersed in Parkinson’s research, reading studies and visiting labs all over the world. He hadn’t taken a biology class since high school, but he learned fast.
“I was able to draw upon my understanding of how to learn a language, and that really helped me dive into the world of biology,” he said.
Stecher started a blog to share his insights into Parkinson’s research with other patients. Some of his readers are where he was when he started: excited about research, but unfamiliar with the day-to-day of basic science. Stecher works to bridge the gap in his blog, Tomorrow Edition, where he posts transcripts of his conversations with experts in all areas of Parkinson’s research.

of Edinburgh was one stop on his “world tour” of Parkinson’s research groups.
For those experts, the anatomists, neurologists and life scientists of every ilk who have been analyzing Parkinson’s disease for the past 200 years, the disease has proved frustrating. A “snowflake disease,” with no two patients looking quite the same, Parkinson’s manifests in an array of motor and non-motor symptoms — including blocked bowels, nocturnal convulsions and a diminished sense of smell — that one patient may experience to far greater degrees than another.
But the symptoms all stem from the aggregation of misfolded alpha-synuclein. Preventing or disrupting its misfolding may be the best chance researchers have for stopping and treating the disease — which has doubled in prevalence over the past 15 years to affect more than 7 million people worldwide.
Before the tremors

Journalist Dave Iverson, who has Parkinson’s, is a patient advisor and contributing editor for the Michael J. Fox Foundation, a nonprofit engaged in funding and advocacy for the disease. At the Parkinson’s Policy Forum, a recent advocacy event sponsored by Fox and the Parkinson’s Foundation, he moderated a panel discussion updating patient-advocate attendees on recent research.
“We used to think that if we could just fix the dopamine supply, we’d be good to go,” Iverson said in his opening remarks. “But now we know it’s a more complicated disease; there are problems with everything from cognition to constipation.”
Almost all patients experience slowing and rigidity of movement, with seven out of 10 exhibiting a tremor at rest in one or more limbs. Many patients also find that their handwriting gets smaller, their voices softer and their faces less expressive. Some develop low blood pressure; others, sleep problems; still others, mood disorders.

Weinschreider, both Parkinson’s advocates from Illinois, at the Parkinson’s Policy Forum, hosted in March by the foundation
in Washington, DC.
Many of these symptoms, including constipation and anosmia, or the loss of sense of smell, develop before the signature tremor.
“Ninety percent of us have no sense of smell,” said A.C. Woolnough, a retired high-school principal from Sandpoint, Idaho, who is a patient and active advocate. “I can’t tell the difference between Coke, root beer and Dr. Pepper.”

Anosmia is caused by the death of olfactory neurons and can have pernicious effects on a person’s quality of life. Edward Burton, a neurology professor at the University of Pittsburgh who treats patients in the movement disorders clinic and also runs a lab, points out that the loss does not just affect one’s enjoyment of food.
“Not being able to smell whether your clothes are clean? Whether you left the gas on?” he said. “These things are kind of disabling.”
Many people think of constipation as a normal part of getting older or eating an unbalanced diet. Few realize that it can be an early warning sign of Parkinson’s development; more than 50 percent of patients suffer from constipation for several years before motor symptoms become apparent.

The seemingly disparate symptoms were linked in 2003, when the German anatomist Heiko Braak published a new model of the disease’s progression.
Braak had performed a series of autopsies on brains comparing patients with symptoms of Parkinson’s to those displaying asymptomatic aggregation of alpha-synuclein into Lewy bodies. Braak and his colleagues noted that Lewy bodies began to accumulate in the anterior olfactory nucleus, which is responsible for processing smells, before they appeared in the substantia nigra, which was known to show neurodegeneration in Parkinson’s. They also found that in some people whose brains had Lewy bodies, motor symptoms never developed. In these cases, the substantia nigra remained intact.

from northern Idaho, wears a shirt promoting
the World Parkinson’s Conference,
an international meeting that brings together
doctors and patient advocates from all over
the world. This stuffed raccoon, the conference
mascot, has hot-pink medical tape around
its paw to show support for research.
The researchers proposed a staged model wherein aggregation of alpha-synuclein started in the olfactory bulb and spread through connections between neurons into the brain. According to the model, when the spread reached the substantia nigra, where dopaminergic neurons are the densest, patients’ ability to move would begin to decline.
In 2007, Braak and his colleagues came up with a new model, known as the dual-hit hypothesis, which introduced the gut as a second possible starting place for alpha-synuclein aggregation.
Both the nose and the gut have abundant nervous tissue, and in both places these neurons are exposed to microbes and other possible aggregation triggers from the environment. The researchers were unsure how alpha-synuclein aggregation passed from one cell to another, and they proposed that a neuron-specific virus might be to blame.
The mechanism remains in question, but in general, the model has held up. Early aggregation of alpha-synuclein in the peripheral nervous system, as proposed in the Braak hypothesis, has become widely accepted as a model for the disease’s progression and appears to occur in about 80 percent of patients, based on autopsy findings.
According to Braak’s hypothesis, if alpha-synuclein pathology begins in the gut, it needs to reach the brain via the vagus nerve, which connects the stomach, intestines and other organs to the brain. Evidence recently gathered from medical records in Denmark and Sweden suggests that patients with a severed vagus nerve developed Parkinson’s at a lower rate than the general population. The procedure, known as a vagotomy, was popular in the 1970s as a treatment for severe peptic ulcers, which later were discovered to be caused by bacterial infection.
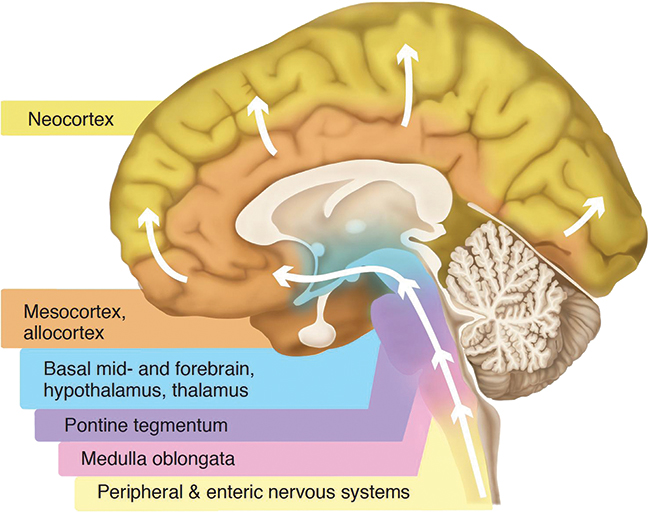
system. The motor symptoms of Parkinson’s disease usually begin when aggregation and neurodegeneration
occur in the substantia nigra, in between the blue and purple regions.
While both the Danish and Swedish studies found that performing a vagotomy led to a reduction in the occurrence of Parkinson’s disease at both five and 10 years after the surgery, the procedure also severs the nerve’s connection to the stomach, liver, gallbladder and pancreas — making it far from practical as a preventative measure. The results of both analyses, however, do provide credence to a role for the gut-brain axis in Parkinson’s disease.

Some researchers believe that disruption to the intestinal wall, perhaps caused by inflammation, may be a factor in triggering alpha-synuclein aggregation. Kathleen Shannon, a neurologist at the University of Wisconsin-Madison specializing in movement disorders, is searching for biomarkers of disrupted intestinal membrane integrity in patients with Parkinson’s.
The U.S. Food and Drug Administration has approved no therapies to halt or disrupt alpha-synuclein aggregation. Shannon and other experts hope to develop treatments that will stop neurodegeneration at early stages. To make that happen, they must identify patients much earlier, before the dopaminergic die-off begins.
“The thought is: Are there things that you can detect in people five to 10 years before onset and intervene then?” Shannon said. “That’s where the whole field is moving right now.”
The genes
If Parkinson’s disease were a product of a single malfunctioning gene, like Huntington’s disease, diagnosing the disorder would be relatively easy, but fewer than 10 percent of Parkinson’s cases have a single-gene cause. Still, recent identification of genetic risk factors may shed light on the various points where aberrant biochemical behavior can give rise to the disease.
“Fifteen or twenty years ago, we didn’t think genetics was involved in Parkinson’s disease at all,” Dave Iverson of the Fox Foundation said.
But genome-wide association surveys and studies of families with a high incidence of the disease have turned up some genetic risk factors. The most common are variants in the lysosomal kinase LRRK2, believed to play a role in the development of 2 percent of all known cases of Parkinson’s, and the lysosomal enzyme glucocerebrocidase A, or GBA, which affects about 5 percent of patients, including Benjamin Stecher.
“We’re coming pretty close to understanding how that mutation actually leads to the disease,” said Stecher, who is participating in research on targeted therapies specifically for patients with the GBA mutation.
Scientists suspect that in cells with pre-existing lysosomal problems, aggregates of alpha-synuclein cannot be cleared effectively.
Aside from lysosomal enzymes like LRRK2 and GBA, some 26 other genes also impart a degree of risk; most do not cause Parkinson’s in every person who carries them, and just one in 10 patients has a known genetic mutation. Experts do not expect to find major new genetic contributors, but research into these mutations ultimately may help every patient by allowing clinicians to extrapolate to treatments targeting shared pathways in the disease.
Some members in the Parkinson’s community are fond of the saying, “The genes load the gun, but the environment pulls the trigger.” While a complex constellation of genetic factors may put a person at risk of Parkinson’s disease, genetics alone cannot account for every case. Experts think that alpha-synuclein begins to aggregate because the environment introduces a stressor.
The twisted protein
Alpha-synuclein typically is found within the presynaptic terminal of neurons and binds to lipids. It may affect the fusion of synaptic vesicles, helping to deliver neurotransmitters to the synapse. It also has been found to misfold and aggregate after viral infection in the brain or traumatic brain injury.
Preliminary results suggest that alpha-synuclein also may play a role in mediating intestinal inflammation.

In a 2017 paper in the Journal of Innate Immunity, Michael Zasloff, an immunologist at Georgetown University, and his colleagues noted high levels of alpha-synuclein in neurons in endoscopic biopsies taken from 42 children with acute or chronic gastrointestinal infections. The researchers believe the protein’s presence during infections suggests a role as part of a gastrointestinal immune response. The researchers also found that alpha-synuclein acted as a chemoattractant for monocytes and neutrophils, essential components of an immune response.
“What we imagine is that in certain settings … where the infection is chronic, and alpha-synuclein is constantly being produced, or in a setting where your genetics is such that the equipment needed to clear alpha-synuclein from the nerve or the surrounding tissue is impaired, alpha-synuclein begins to accumulate,” Zasloff said. “And when it accumulates, for example in the nerve (cells), you start to see pathology.”
Levels of alpha-synuclein expression are highest in the dopamine neurons of the substantia nigra, which is why they are thought to be most sensitive to the prion-like spread of alpha-synuclein aggregation. However, it isn’t clear quite how the problem goes from a single fibril to a systemic invasion — whether, for instance, aggregates get deposited by dead or dying cells and taken up by other cells.
“I think everybody in the field more or less agrees that there are intrinsic factors in some neurons that make them more susceptible (to alpha-synuclein aggregation),” said Burton, the Pittsburgh-based physician-scientist. “The question is whether the temporal cascade of pathology is spreading physically, or whether there is a systemic problem that affects each population of neurons in order of their susceptibility.”
In either case, the first fibril of alpha-synuclein must form somewhere, and researchers have identified potential culprits.
“If environmental factors involved in Parkinson’s disease trigger alpha-synuclein pathology, the gastrointestinal tract is a really good candidate for the site of initial aggregation,” Burton said. “The gut presents a huge surface area that could be exposed to environmental toxicants.”
The environment
Researchers have identified an array of molecules, including pesticides and bacterial byproducts, that can trigger symptoms of Parkinson’s in animal models. While it is not clear why giving each of these molecules to rodents induces alpha-synuclein aggregation, they reliably cause both Lewy body formation and motor symptoms similar to those observed in Parkinson’s patients. Several mechanisms have been proposed.
“It’s quite possible there are multiple different ways you can enter the pathophysiological cascade” that leads to spreading alpha-synuclein pathology, Burton explained.
Some of the chemicals also have been linked to the disease in humans.
One such pesticide, paraquat, is a precursor to a neurotoxin that resembles dopamine. Once metabolized into the neurotoxin, the molecule enters cells through a dopamine transporter and kills them.
Another precursor to the same toxin is a chemical byproduct of opioid synthesis. Its neurotoxic effect was discovered after a drug user, who was also a graduate student in chemistry, injected himself with an impure home brew and developed Parkinson’s symptoms just days later. The man suffered from Parkinson’s for the rest of his life; an autopsy found alpha-synuclein aggregates in his brain.
Another pesticide, rotenone, was commercially available until an epidemiological study published in 2011 showed that exposure to it increased farm workers’ risk of Parkinson’s. Although rotenone, which blocks mitochondrial respiration, is in principle available to every cell in the body, dopaminergic cells seem the most sensitive to the stress of coping with the toxin.
Man-made compounds in the environment may not be the only culprit.

In a 2016 study published in the journal Cell, researchers from Sarkis Mazmanian’s lab at the California Institute of Technology showed that while overexpression of alpha-synuclein can predispose mice to Parkinson’s, keeping the mice in germ-free conditions protected them.
“If mice received microbiota from a human donor, they had worse motor symptoms than mice that received microbiota from a health control,” Mazmanian said. These results suggested that a bacterial product in, or from, the gut might cause the first seed to form in a host cell.
A variety of bacterial products have been proposed. One possibility is that one or more prion-like bacterial proteins may act as a seed, a hypothesis put forth by Robert Friedland, a neurologist at the University of Louisville, in the Journal of Alzheimer's Disease in 2015. Alpha-synuclein is just one of many proteins, termed amylogenic, that tend to misfold and aggregate into amyloid fibrils. Matthew Chapman, a microbiologist at the University of Michigan who began collaborating with Mazmanian’s group after their 2016 paper, studies proteins in bacteria that share many of alpha-synuclein’s structural properties.

“It’s possible that functional amyloids made by things in the microbiome could ‘seed’ human proteins,” said Chapman, who has also collaborated with Friedland. “Therefore, it is possible that a seed provided by a bacterial functional amyloid could be the trigger or nucleator.”
In 2016, Friedland and colleagues found that rats that were overexposed to bacteria producing amyloid fibers displayed an increased accumulation of alpha-synuclein in both the brain and the gut.
Other researchers, including a South Korean team headed by Myung Sook Oh, have proposed that a non-protein molecule from bacteria in the gut may trigger alpha-synuclein aggregation. Lipopolysaccharides are a broad class of sugar-linked lipids from the outer membrane of many bacteria and are well-known triggers for inflammation. Biochemical experiments showed that when lipopolysaccharides were mixed with purified alpha-synuclein, toxic fibrils formed more readily than they would in a solution of alpha-synuclein alone.
Lipopolysaccharides also have been shown to trigger Parkinson’s in animals, potentially due to long-term low-grade neuroinflammation after they are introduced to the eye or the gut. Oh’s lab reported in January in the journal Scientific Reports that in several pesticide-induced mouse models of Parkinson’s, they found upregulation of a single gut microbe, Proteus mirabilis.
The researchers isolated lipopolysaccharide from the bug and added the molecules to mice that had been exposed to a pesticide level previously too low to develop Parkinson’s symptoms. The addition caused the mice to develop symptoms.
Likewise, Mazmanian’s group found that mice bred to over-express alpha-synuclein could be pushed to develop Parkinson’s symptoms by receiving a fecal transplant from a Parkinson’s patient.
However, their study also found that feeding the mice a sterile mixture of three bacterial metabolites known as short-chain fatty acids could trigger the motor and non-motor symptoms of the disease — even though, unlike lipopolysaccharide, the short-chain fatty acids could not seed aggregation independently.
These metabolites are not exotic; every person’s gut has microbes that make short-chain fatty acids. This finding also appears to be at odds with studies in humans demonstrating that short-chain fatty acids are helpful, not harmful, in the onset of Parkinson’s (see: “The short-chain conundrum”).
Interestingly, Mazmanian’s group also found that germ-free mice that received a specific mixture of short-chain fatty acids seemed to have neuroinflammation triggered by the activation of microglia in the brain — another role for the molecules at odds with their regular function.
Brain inflammation
The main immune cells governing neuroinflammation are microglia, a strain of glia that are derived from the same stem cells as macrophages. Like macrophages throughout the body, microglia are tissue-resident cells that can sense infectious agents and eliminate them through phagocytosis.

Marina Romero–Ramos, a neurobiologist who studies microglia at Aarhaus University in Denmark, noted that in animal models of Parkinson’s disease, activated microglia cluster in areas where Lewy bodies are especially dense. This immune response mimics the response found in autopsies of Parkinson’s patients and seems to suggest that the microglia are responding to the neuronal event initiated by alpha-synuclein, she said.
“For many years, it was thought that microglial activation came as a consequence of initial cell death of neurons,” Romero–Ramos said.
In this classic view, microglia were activated by dying neurons and responded harshly with a neuroinflammatory response that did further damage to surviving cells. However, researchers now know that microglial activation starts much earlier. Romero–Ramos said that, because they constantly survey the tissue around them, microglia respond early to very small changes in neurons. In other words, they may get involved before neurons begin to die.
Mazmanian’s study did not examine whether microglia were activated directly by the bacterial metabolites; they may have been responding to immune events initiated by the metabolites elsewhere in the body. But Romero–Ramos allows that, because the molecules are lipid-soluble, “in theory, (SCFAs from the gut) could reach any cell in the body. They could reach neurons, or they could reach immune cells.”
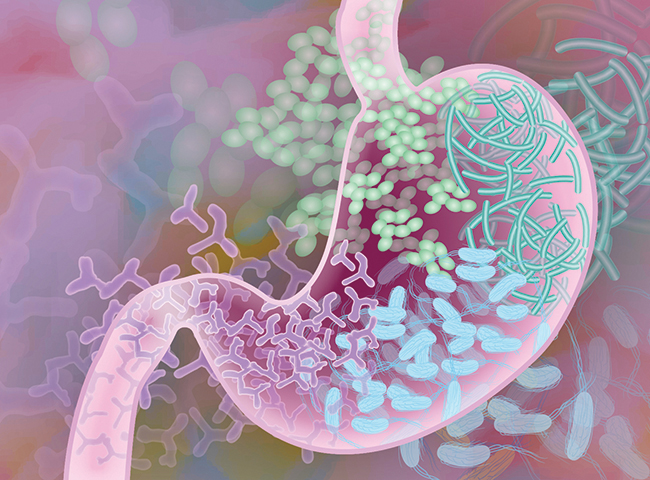
microbe, or microbes, that may be responsible for the aggregation of alpha-synuclein in the human gut.
Inhibiting microglia using a small molecule, minocycline, reduced inflammation in the brain and delayed the onset of Parkinson’s-like symptoms in Mazmanian’s study. So, should inhibition of microglia be regarded as a promising treatment for Parkinson’s? It’s a complicated question, Romero–Ramos said.
Research by prominent glia biologists in the last decade has shown that microglia are key contributors to synapse pruning and neuronal circuit formation during development and also may be important for cognition in adults. And even when activated and releasing cytokines, the protein factors that drive inflammation, the immune cells can release pro-survival factors that keep neurons alive. Moreover, a study of how cells degrade external alpha-synuclein aggregates found that microglia can remove and destroy the aggregates more efficiently than any other brain cell type.
“Many of the studies up to now were very focused on quieting microglia, and that has proved not too successful ... I think it’s because we knew very little about the microglia,” Romero–Ramos said. She believes that future Parkinson’s treatments will modulate microglial activity, but that before such treatments can be developed, we need to understand the cells better.
“In the future, most likely, treatments will combine something that is focused on neurons, with probably a second drug that is focused on the immune cells as well,” she said. “Unless we really find the cue to why alpha-synuclein aggregates, I am pretty sure that our therapy will be a combined therapy.”
The search for a drug
As researchers propose possible future treatments, the Parkinson’s patient community is instrumental in helping test these therapies in clinical trials. By Woolnough’s count, he has participated in 21 studies.
“If I see something that I’m eligible for and that fits my timeline, I’m ready to jump all over it,” the retired high school principal said. “I’ve got two sons and three grandsons. That’s why I do it.”
Woolnough likes to say that Parkinson’s gave him a part-time job to keep busy in retirement. Besides participating in research, he writes a column for a local newspaper and serves as a patient member of grant review committees for the Parkinson’s Foundation and the Department of Defense. Last year, he joined fellow patients and a few llamas on a fundraising hike, an endeavor he wryly refers to as “60 miles on the Pacific Crest Trail that nearly killed me.”
He has volunteered for a number of observational trials, which aim to deepen understanding of the disease without interfering with the ordinary course of treatment. One research group sent him an accelerometer-enhanced smart watch to monitor his tremors continuously from home. Another took a spinal tap, blood samples, and biopsies from his salivary gland and colon to establish whether alpha-synuclein aggregates reliably appear outside the brain during disease, and if so, where within one person a diagnostic should be targeted.
Woolnough isn’t involved in any tests of new drugs at the moment. But several clinical trials for Parkinson’s are underway, some aimed at improving quality of life and others testing interventions that scientists hope will stop the spread of alpha-synuclein aggregates. These include immunization or monoclonal antibodies against alpha-synuclein. Another trial, due to be completed in 2019, tests a calcium channel blocker that currently is used to control blood pressure, on the hypothesis that it may protect dopaminergic neurons.
In contrast to therapy to block the progression of Parkinson’s, which has proved elusive, new therapies to improve patients’ quality of life have become increasingly available. For example, several supplementary drugs to treat side effects of levodopa, the most commonly prescribed drug for Parkinson’s, became available last year.
Many molecules have failed, however, at disease modification — so many that some major pharmaceutical companies are cutting their investment in research and development for neurodegenerative disorders. Earlier this year, Pfizer announced that it would drop funding for its neurodegeneration programs; AstraZeneca sharply reduced its neurosciences division in 2016.
Some of this disinvestment is informed by failures of drugs to treat Alzheimer’s disease, which is more common than Parkinson’s and has a similar neurodegenerative mechanism driven by protein aggregation. Over the last two decades, Pfizer sponsored close to 100 clinical trials for 24 potential drugs to treat Alzheimer’s, only one of which ultimately was approved. That drug, Aricept, is used to treat the dementia that the disease brings, rather than targeting the beta-amyloid plaques responsible for neurodegeneration.

Despite this news, Gregory Petsko, a biochemist at Weill Cornell Medical College, is hopeful about the future of Parkinson’s treatment.
“In a sense, this is easier to deal with than cancer,” he said, “You’ve got to kill every cancer cell, or you know what could happen. But, here, if you delayed the onset of Parkinson’s disease by 20 years, on average, we wouldn’t be having this conversation, because this would no longer be a major health problem.”
A handful of startup drug companies are looking to tackle Parkinson’s disease in the gut.
Enterin, a pharmaceutical company Georgetown’s Zasloff founded in 2014, recently concluded a clinical trial assessing the safety and tolerability of a synthetic squalamine derivative, ENT-01, in patients with Parkinson’s disease. Squalamine is an antimicrobial originally isolated from dogfish sharks in 1993 in Zasloff’s lab. The company hopes to show that ENT-01 is useful for treating Parkinson’s-related constipation.
Like squalamine, ENT-01 prevents aggregates from forming in purified alpha-synuclein samples. In cultured cells, squalamine has been shown to displace aggregates of alpha-synuclein from the lipid membranes of neurons. In a model of Parkinson’s disease in the roundworm Caenorhabditis elegans, it was shown to inhibit the formation of toxic alpha-synuclein aggregates and prevent the onset of paralysis.
Zasloff believes the alpha-synuclein that aggregates in the gut is a product of the body’s immune response to gastrointestinal infections.
“In the normal, day-to-day life of the gut’s nervous system, it is expressing alpha-synuclein to either protect itself or the local milieu that it lies within, within the wall of the gut, and it does so by calling in a variety of white blood cells and by alerting the immune system,” Zasloff said.
In an unusual move for a trial testing an intervention for neurodegeneration, Enterin’s clinical trial did not try to measure neurodegeneration or changes in movement among patients. Instead, it focused tightly on the gut, looking for a change in constipation.
“The trial was meant to test the hypothesis that (the squalamine derivative) could reverse the functionally inactive enteric nervous system of an individual with Parkinson’s,” Zasloff said.
The investigators also watched for effects of ENT-01 on the motor symptoms, mood changes, sleep disruptions and hallucinations associated with the disease.
Data from this clinical trial are still being analyzed. Zasloff and his colleagues are planning a more rigorous clinical trial early next year, in which a larger group of patients with severe constipation will receive the drug for several months.
This isn’t the first time Zasloff has gone through clinical trials with derivatives of squalamine. His first drug company, Genaera Corp., founded in 1989 as Magainin, conducted, but ultimately halted clinical trials exploring squalamine’s ability to treat prostate cancer and a severe form of macular degeneration by blocking blood vessel growth.
Zasloff is optimistic that squalamine can be used to treat a number of complex neurological conditions, including schizophrenia and autism; he and Enterin are slated to begin early trials with ENT-01 for both disorders in 2019.
Mazmanian, at Caltech, also has a startup exploring the role of the gut-brain axis in Parkinson’s disease. In 2013, Mazmanian helped found Axial Biotherapeutics, based on earlier work from his lab that attributed autism-like symptoms in mice to a deficiency of either the bacteria Bacteroides fragilis or its metabolites. Last fall, the company’s CEO stated that they intend to bring at least one therapy for autism to early clinical trials this year, and the company has indicated that it is developing a Parkinson’s therapy based on work from Mazmanian’s lab. Like Enterin, Axial aims first to measure the effects of its interventions on patients’ GI function rather than the movement symptoms that are more typically used to measure the disease.
While drug companies inch forward, patients have attempted to take treatment into their own hands with nutrition — and less savory actors have sought to take advantage of them.
Nutrition and snake oil
“When I go to my neurologist — and I know this is the case for the vast majority of patients—there’s no talk of nutrition,” Stecher said.
Doctors rarely make nutritional recommendations to patients with Parkinson’s, and reputable patient-education groups issue the mildest of healthy-eating guidelines, recommending a balanced diet with plenty of fiber and water.
Stecher rattled off a list of supplements, including vitamin D and coenzyme Q10, that have been tested based on promising preclinical data but failed to show a consistent effect in patients.
“There are so many things that have been associated with having a neuroprotective effect,” he said. “But actually identifying which ones are truly neuroprotective and which ones people should be taking? We’re not there yet.”
Epidemiologists have found some habits, such as cigarette smoking and coffee drinking, that dramatically reduce the risk of developing Parkinson’s disease. However, large-scale clinical trials testing whether nicotine or caffeine could help patients proved futile.
There may be no clear data on proper nutrition with Parkinson’s, but a wealth of questionably sourced diet books purport to have the answers, according to Michael Okun, the chair of the University of Florida’s neurology department and the medical director of the Parkinson’s Foundation.

“Just like every disease, there’s a cottage industry,” Okun said. “The harsh reality in Parkinson’s disease, in my opinion, is the businesses have popped up before the data and the science is really there.”
Plenty of patients, credulous or desperate, don’t want to wait for randomized controlled trials. In A.C. Woolnough’s support group, he said, patients often pass along rumors about supplements or alternative treatments.
“I say, ‘You know what? Show me a randomized double-blind study with a placebo, (and) I might take your word for it,’” he said. “It may be perfectly true. But can it be replicated?”
Okun takes a similar attitude. Much of his time both in the clinic and on the Parkinson’s Foundation website, where he runs an “ Ask the Doctor” forum, is devoted to pointing patients toward reliable sources of information. He acknowledges that patients eager to try alternative therapies may be on to something, but cautions that they may be taken advantage of.
“There’s oftentimes a sales pitch that goes with (an alternative therapy), that you’ve got to keep going with the vitamins or keep going with the nutraceuticals,” he said. “We’ve seen people drain their savings.”
A bag of chemicals and cells
Scientists have their work cut out for them determining why alpha-synuclein misfolds and how it can be prevented. Mazmanian and his colleagues are currently sharpening their focus on single microbes that may cause the onset of sporadic cases of Parkinson’s.
In the meantime, researchers are walking a high-wire, balancing optimism for the possibilities of new treatment on one side with overselling their findings on the other.
“Because there’s nothing for these diseases, this field is really susceptible to fads, so many things have failed, right?” Petsko said. “Anything new that comes along, people jump on like crazy in the hope that it might work. That sounds like a criticism, but it isn’t.”
that triggers misfolding of the protein.
But the research enterprise itself is fascinating. Just ask Stecher.
While he writes his blog primarily as a service to other patients, the work seems to do Stecher a lot of good, too. “Even though Parkinson’s itself sucks, and daily it can be quite a hassle, I am somewhat thankful that it’s opened my eyes to the world of biology.
“At the end of the day, we are just a bag of chemicals and cells and proteins that kind of chaotically interact with the environment and the world around us. Prior to being diagnosed with Parkinson’s and delving into all this research, I had no real basis for appreciating that. Now that I do, it’s something that fills me with awe and wonder.” 
The shaking palsy

The constipation, disrupted sleep and creeping decline in motor function that mark the disease were first described in 1817 in a monograph by the English surgeon James Parkinson, who called it “the shaking palsy.”
Nearly 100 years later, the neurologist Frederic Lewy discovered that proteins aggregated in the neurons of Parkinson’s patients. These spherical aggregates were later found to be responsible for the death of dopamine-signaling, or dopaminergic, neurons in the substantia nigra, a part of the mid-brain involved in motion and reward.
In 1957, the Swedish scientist Arvid Carlsson found that when dopamine was depleted from rabbit brains, a Parkinson’s-like phenotype could result. The disease could be reversed by administering levodopa, which was approved for human Parkinson’s patients in 1967. The drug, which acts by replacing a patient’s depleted dopamine, remains the most widely prescribed treatment for the disease, but does nothing to halt the march of neurodegeneration. Levodopa, a mirror image of dopamine, crosses the blood-brain barrier, an obstacle that made direct dopamine replacement impossible. Once in the brain, it rapidly converts to dopamine, which reduces tremors and shakes and improves a patient’s motor control. However, levodopa’s quick metabolism gives patients only a brief window of relief before symptoms return.

Ben Stecher, who was diagnosed with Parkinson’s at age 29 and who blogs about the disease, started taking levodopa after milder drugs for tremor, such as dopamine breakdown inhibitors, didn’t work for him.
“The on/off of levodopa has become the metronome of my life,” Stecher said, describing an “on” that can last from 20 minutes to two hours before slipping away. “When I’m off, I feel as though I’m stuck in the mud, not just my body, but to some extent my mind as well.”
Over the long term, levodopa can cause dyskinesia, a clinical term for jerky, involuntary movements. And although replacing the missing dopamine can relieve symptoms, it does nothing to prevent further spread of alpha-synuclein throughout the brain.
A less-common treatment for late-stage Parkinson’s is deep-brain stimulation, in which micro-electrodes are implanted into one of three regions of the brain’s basal ganglia, depending on symptoms. Peggy van Hulsteyn, a writer living in Santa Fe, was diagnosed 18 years ago and had DBS surgery in 2009.
“I have to admit, when I first heard about DBS, I was horrified,” van Hulsteyn said. “My steadfast and affable neurologist, Scott Sherman, was the constant cheerleader for this scary surgery.”

Looking back, van Hulsteyn describes the surgery as the best thing she’s done to manage her Parkinson’s. DBS does not work for every patient and can be risky especially for older patients; however, in patients for whom it works, the suppression of motor symptoms with DBS compared to levodopa alone can last for 10 years or more.
The implanted micro-electrodes are connected by a subcutaneous wire to a battery-powered pacemaker placed below the collarbone. With an external remote control, a patient can turn the system on or off once it has been programmed by their physician. The system can complement levodopa treatment, allowing a patient to take smaller doses of the drug, and it counteracts some of the dyskinesia that can occur with higher doses. DBS can be an effective treatment for late-stage Parkinson’s, but like levodopa, it has no effect on the progression of alpha-synuclein.
A short-chain puzzle
Acetate, propionate and butyrate, the three short-chain fatty acids Sarkis Mazmanian’s Caltech lab added to the water of germ-free mice, are products created when microbes break down dietary fiber. In that study, feeding germ-free mice predisposed to Parkinson’s disease short-chain fatty acids tended to trigger Parkinson’s-like symptoms.
Studies in humans show an inverse correlation between levels of short-chain fatty acids and Parkinson’s and generally find a reduction in levels of the molecules in stool samples from people with the disease. Fiber, which is broken down into short-chain fatty acids, has been proposed to play a role in the lower age-specific prevalence of Parkinson’s disease in Africa and India, where consumption of vegetables is higher. Some work has linked butyrate to neuroprotection and diminished symptoms in mouse and fly models of Parkinson’s, though early clinical studies of a butyrate derivative for Parkinson’s patients have no reported success.
Acetate appears to be the odd chain out. While butyrate and propionate are known to activate a subset of G-protein coupled receptors, inhibit histone deacetylases and suppress inflammation through regulatory T cell, acetate doesn’t share these roles. Short-chain fatty acids are known to promote intestinal contraction and affect immune signaling and gene expression.
The contradiction between Mazmanian’s finding and other studies may stem from varying amounts of the short-chain fatty acids, which can be made in different proportions by different microbes.
Parkinson’s in women
Another quirk of one of the most complicated human diseases turns out to be its higher incidence in men than in women. On average, men have 1.5 times the risk of developing Parkinson’s as women, and women tend to develop Parkinson’s two years later than men; however, no definitive explanation exists for this disparity. One hotly debated hypothesis involves levels of estrogen, which are believed to play a role in dopamine synthesis and help modulate dopamine receptor function. Other hypotheses involve genetic risk factors that are X chromosome–linked, which makes the genes more likely to be inherited in men. Disparity in careers that put workers at a higher risk of head trauma or increase exposure to environmental toxins may also be to blame.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.






