
Cannabis hyperemesis and the cure that burns
At a remote U.S. Army outpost in Afghanistan sometime in 2019, a defense contractor began to vomit uncontrollably.
Medics gave him intravenous fluids and a drug for nausea. It didn’t help. Unable to get the man’s symptoms under control, the doctors had him medically evacuated to Bagram Air Base, which housed the most sophisticated military hospital in the country.
There, Rory Stuart, a lieutenant colonel in the U.S. Air Force and an emergency physician at the University of California, Davis, worked the patient up. The man kept vomiting and retching while Stuart’s team conducted tests that proved inconclusive. His vital signs were normal. Scans and samples of his blood and urine showed no appendicitis, no kidney stones: just intestinal inflammation and kidney problems possibly caused by severe dehydration. Figuring the patient might have a particularly nasty viral infection, Stuart admitted him to the hospital for observation and more fluids.

in Kuwait in 2018, brought Rory Stuart’s patient from a remote outpostto Bagram
Air Base. The flight was not without risk for the pilots and crew, Stuart said.
A few hours later, Stuart received a call from a colleague who supervised the inpatient unit. Stuart recalls his colleague saying, “‘He’s freaking the other patients out, he’s making the nurses mad, and it’s just so weird: He won’t get out of the shower.’”
Suddenly it all made sense. “I was like, ‘Oh, God, are you kidding me?’ Because I had dealt with these patients back home,” Stuart said.
He asked the nurses to collect a second urine sample. Unlike the first sample, this one was high in sugar and had the wrong specific gravity. It also, lab technicians observed, smelled an awful lot like the apple juice the patient had been served at lunch.
By testing the original urine sample again, the technicians found exactly what Stuart was expecting: a high level of tetrahydrocannabinol, or THC, in the patient’s system.
The experience, which Stuart and a UC Davis colleague wrote up in the journal Military Medicine, was an unusually colorful instance of cannabis hyperemesis syndrome. The ailment afflicts a small subset of heavy cannabis users, causing relentless vomiting after exposure to the drug — the opposite of what most users expect. The hot showers, a widely used home remedy, hint at a fascinating molecular crosstalk that physicians still do not understand.
A paradoxical diagnosis
Just about everyone knows that cannabis can prevent nausea and vomiting, especially among cancer patients undergoing chemotherapy. So cannabis hyperemesis syndrome, or CHS, is counterintuitive.
One study found that, on average, patients visit a doctor’s office or emergency room three to five times before they receive a diagnosis of CHS. “These patients experience the worst of medicine,” said Jeff LaPoint, an emergency toxicologist. Often in terrible pain, they go through many scans to rule out emergencies, such as appendicitis or ectopic pregnancy, and more slowly developing illnesses that require medical interventions. Before the vomiting starts, people often have weeks of stomach pain or nausea — both of which, like vomiting, could have any number of causes that must be ruled out. One Reddit user, interviewed by text for this story, said they knew more than one person who had had their gallbladder removed in a fruitless effort at resolving their illness.
Perhaps that’s why, by the time a doctor tells a patient that cannabis is the cause of their misery, patients rarely accept that explanation. No, they’ll explain: They’re using the weed to relieve the nausea. Stuart, the air force physician, said, “You rarely win that argument.” Yet a core diagnostic criterion for CHS is that when people stop using the drug, their symptoms resolve.
The particulars of pot and puking
Vomiting depends on a part of the hypothalamus called the nucleus tractus solitarius, which integrates many inputs. It connects to parts of the brain and peripheral nervous system that sense movement and position, chemicals circulating in the blood, and the status of the stomach and gut. It also takes input from the cortex, where higher-order thinking takes place.
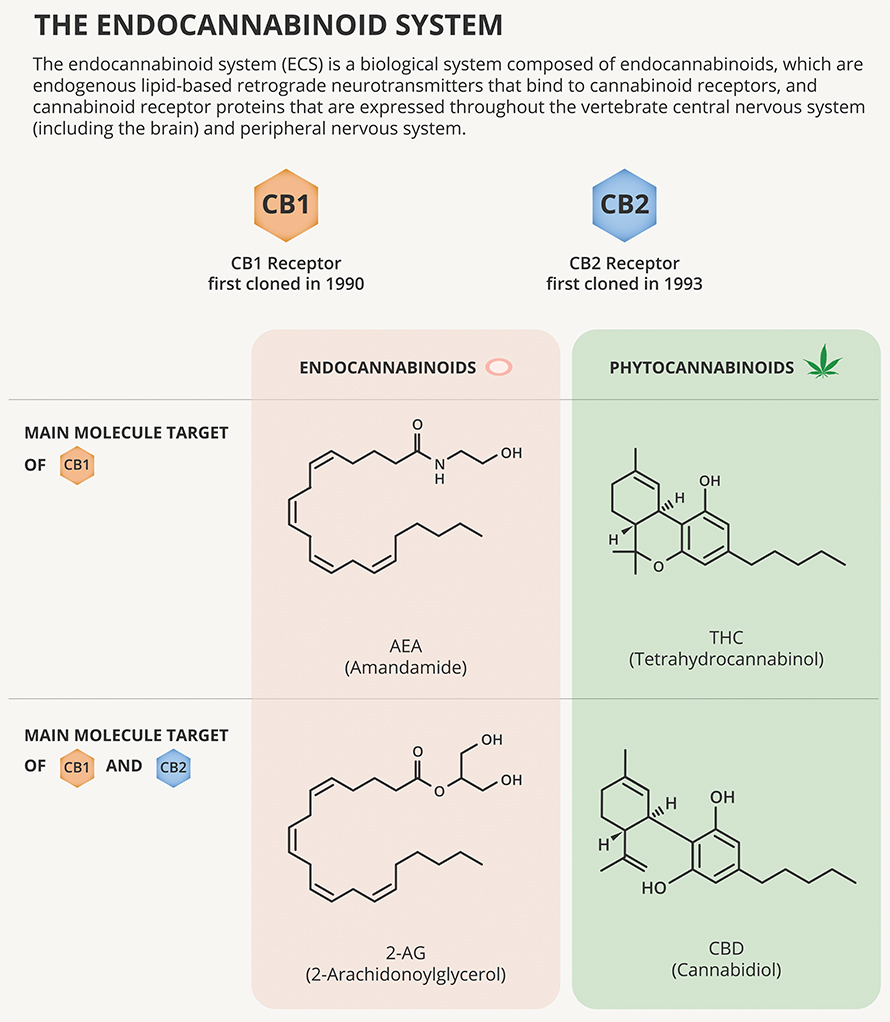
According to Vincenzo Di Marzo, a biochemist who studies endocannabinoid and cannabinoid signaling at Quebec’s Université Laval, most of the neurons in the vomiting center and its chemical-sensing trigger zone express the cannabinoid receptor CB1R. The most abundant G protein–coupled receptor in the brain, it responds to signaling molecules called endocannabinoids.
When CB1R at the presynaptic terminal of a neuron is activated, it makes that neuron less likely to fire at that synapse. CB1R also is expressed in the peripheral nervous system in the vagus nerve and enteric nerves that connect the brain with the gut.
The most abundant compound in marijuana, THC, causes a user to get high by binding to CB1R. In animal models, that interaction also can make vomiting less likely.
A second cannabinoid receptor, called the CB2 receptor, also binds to THC and endocannabinoids. CB2R is expressed mostly in immune cells, but researchers led by University of Alberta scientist Keith Sharkey reported in 2005 that this second set of cannabinoid receptors does appear in neurons in the vomiting center, and stimulating them also reduces vomiting.
Why, then, does a frequent high dose of cannabis cause vomiting?
“Sometimes, drugs produce exactly the opposite effect of what you’d expect,” Di Marzo said.
Because CHS mostly arises among people who have used cannabis at least once a week, often for years, and because cannabinoids tend to accumulate in tissues, there is reason to surmise that the syndrome results from desensitization of cannabinoid receptors, especially CB1R. Desensitization through phosphorylation, arrestin binding or internalization is a common biochemical response to restore homeostasis after chronic receptor activation.
Ethan Russo, a neurologist and co-founder of a cannabis-focused biotechnology company called Credo Science, said that any CB1R agonist can cause cannabis hyperemesis syndrome. For example, people using synthetic cannabinoids, which activate the CB1 receptor very strongly, can become ill even without taking THC.
Di Marzo said that if baseline activity from CB1 receptors is necessary to keep the vomiting center quiet, then desensitizing those receptors could be a trigger for CHS symptoms.
In recent years, marijuana products have become much higher in THC than they used to be, and experts suspect that change, combined with increasing state legalization nationwide, has spurred an increase in CHS cases since the condition first was identified in 2004.
“It’s like if we were drinking Newcastles for 4,000 years, and then one day we decriminalized that and said, ‘Here’s Everclear; it’s the same thing,’” said Jeff LaPoint, the director of the medical toxicology division at Kaiser Permanente in San Diego.
A predilection for hot showers
The defense contractor in Afghanistan responded very typically to the treatments his doctors tried. First, he showed no response at all to the first drug to treat nausea and vomiting, or antiemetic, that the doctors tried, an emergency department workhorse called ondansetron.
Michael Mullins, an emergency toxicologist at Washington University in St. Louis, said, “We’ll often give it in the waiting room, because it’s so safe.”
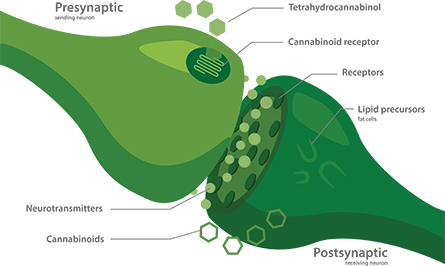
Bundles of neurons relaying messages from different sources to the vomiting center use different neurotransmitters and neuromodulators to communicate. Therefore, many antiemetic drugs can work in some instances but not others. According to U.K. pharmacologist Gareth Sanger, who helped to develop the drug, ondansetron works by blocking a group of serotonin-gated ion channels found in the vagus nerve, which connects the stomach to the brain.
Although patients get no relief with a first-line anti-nausea drug, many experiencing CHS find that a hot shower can help ease their symptoms. The first case series that described the syndrome noted compulsive bathing as one of its patients’ signature characteristics. Physicians say that they don’t see the same degree of devotion to hygiene among other patients in gastrointestinal distress.
As a medical fellow in New York, LaPoint treated some of the first people to arrive in city hospitals with synthetic cannabinoid poisoning. He also happened to have been writing a textbook chapter on cannabinoids. The juxtaposition got him thinking. Because hot baths work so well — but are an impractical treatment for emergency room patients — LaPoint said that many in his field were trying to figure out easier ways to help CHS patients warm up.
LaPoint wondered, while mulling it all over, whether TRPV1, one of the body’s best-known heat-sensing receptors, might be involved in the relief that hot bathing can bring. Not long after TRPV1 was discovered — a finding that earned the 2021 Nobel Prize in Physiolgoy or Medicine — scientists had shown that TRPV1 is also part of the endocannabinoid system. The receptor binds to the endocannabinoid anandamide and to a number of botanical cannabinoids, although not THC.
LaPoint still remembers the first patient he tested the idea with. She was an 18-year-old who used cannabis concentrates known as dabs. She had been in and out of the hospital around five times in the past week. She arrived in such pain that her screams preceded her down the hall. LaPoint found a woman security guard and sent the patient to the doctors’ shower to get the pain under control. The remedy worked, but as the patient waited for her ride to arrive, her pain came back.
LaPoint suggested an off-label application of capsaicin cream. The cream, which uses a molecule from chili peppers to provoke a sensation of heat by activating TRPV1, was safe and widely used to diminish arthritis pain.
“Look, no one has ever done this before,” he recalled telling her. “I wouldn’t ask you about this when you’re screaming in pain — but now you’re just mildly uncomfortable.”
The patient was game — and the cream, applied to her abdomen, seemed to help. It was a breakthrough, LaPoint said, “to have an over-the-counter medication that someone could take home and be functional.”
LaPoint reported at the 2014 North American Congress of Clinical Toxicology that applying capsaicin cream to the bellies of seven patients with CHS resolved their symptoms within half an hour.
Presented with a remedy for these patients, who were otherwise difficult to help, other emergency toxicologists tried it too. Mullins at WUSTL and colleagues published a retrospective case series in 2017 showing that almost all of 13 patients who had received serotonin antagonists to no effect improved after using topical capsaicin. But some colleagues found the concept dubious, critiquing the lack of a plausible mechanism of action.
A team at the University of Virginia currently is conducting a clinical trial comparing the effects of capsaicin to a placebo. The investigators expect to complete the study this year.
Capsaicin is by no means the only treatment toxicologists have tried out for CHS patients. The medical literature has a long list of case reports wherein doctors describe testing an antiemetic in one or a few patients with CHS.
When capsaicin isn’t available — like at military hospitals, which rarely see arthritis patients — physicians can instead use a dopamine antagonist such as droperidol or haloperidol. That’s how Rory Stuart treated his patient in Afghanistan. Within two days of starting IV fluids and haloperidol, the man left the hospital.
Dopamine antagonists have their drawbacks — they can sedate a person, making it unsafe to send them home from the ER by car, and they carry a risk of cardiac side effects. Still, Mullins called droperidol “one of my favorite drugs in the whole wide world, because it almost never fails for nausea and vomiting.”
Between capsaicin and droperidol, some emergency physicians see the CHS problem as solved. Still, questions linger around the disease’s pathophysiology and its unusual treatments.
Many cannabinoids, many receptors
Assuming capsaicin works as well in a placebo-controlled trial as emergency toxicologists think it will, one question is why activity of its target, TRPV1, is important.
TRPV1 is expressed both in the vomiting center in the hypothalamus and in the vagus nerve, and its ligands tend to reduce vomiting in animal models. In fact, capsaicin has been used to block vomiting that’s a side effect of chemotherapy. But according to Di Marzo, researchers don’t know whether its activation or desensitization is driving a reduction in vomiting.
In a review article on CHS in the journal Clinical Toxicology, researchers ticked through numerous reasons that activating TRPV1 might be effective: Perhaps it diverts blood flow to the skin to cool down, reducing pain in the gut. Perhaps its activation blocks the transport or release of neuropeptides from pain-receptive nerve fibers to the hypothalamus. Perhaps the pain from capsaicin simply overrides pain sensed in the gut.
The endocannabinoid system is notoriously difficult to study because its many receptors overlap in function. Cannabis complicates matters further, with a cornucopia of closely related molecules, each of which affects several receptors. THC and CBD may be the best known and the most abundant active compounds in a cannabis leaf, but they are two among many, including cannabigerol, cannabidiolic acid and dozens more. Several of those secondary cannabinoids, Di Marzo’s lab has shown, are also TRPV1 ligands, although none seems to open the channel as strongly as capsaicin does. Different strains of cannabis and different preparation methods can alter the cannabinoid profile of a dose of smoke, edibles, oil or dab.
According to University of Pennsylvania structural biologist Vera Moiseenkova–Bell, cell signaling may be disrupted at several points by the presence of a high concentration of cannabinoids. Cannabinoids are hydrophobic. They’re at home embedded in the plasma membrane, where they can slip into binding pockets buried in the transmembrane domains of many receptors.
As membrane-spanning protein structures have become easier to study thanks to new biophysical methods, researchers have been able to zero in on how cannabinoid–receptor interactions work — and even have begun to probe how cannabinoids alter receptors’ interactions with other ligands.
Working with the ion channel TRPV2, Moiseenkova–Bell and her lab recently published a structural study that showed exactly where CBD and the channel’s canonical ligand, 2-ABP, bind. The lab also found that when they both bind at the same time, they produce a stronger response than either alone.

Whether CBD could boost capsaicin activation on TRPV1 in the same way is not known, Moiseenkova–Bell said — but such drug–drug interactions are possible.
To sort out which cannabinoids are important in the development of CHS, Rachel Wightman, an emergency physician and medical toxicologist at Brown University, is conducting a mixed-methods study. It’s the only study of CHS that the National Institutes of Health currently is funding.
Wightman’s research team is recruiting patients who arrive at the hospital with presumed CHS. They draw blood for metabolomic testing to compare the presence of cannabis metabolites when patients have symptoms to when they do not.
They also are looking to understand the patterns of the disease and cannabis use practices through surveys and interviews. How often do episodes of severe vomiting arise? How do patients feel about the care they receive and the recommendation that they stop using?
Another salient question is what differentiates people who develop CHS from peers who use a similar amount of the drug but never become nauseated.
After running a small genetic study comparing 28 people who were experiencing CHS to a dozen who never had it, Ethan Russo and colleagues at CReDO Science identified a group of genetic variants that appear enriched among people with CHS.
They found single-nucleotide polymorphisms in genes that code for enzymes that break down cannabinoids and farther afield, including two genes involved in dopamine signaling, a lipid transporter protein and a noncoding region of the genome close to the TRPV1 gene.
The study identified correlations only, leaving further causal explanation up to future research. Russo’s company has begun to sell a genetic test based on those findings, but the Food and Drug Administration has not evaluated its ability to screen for a predisposition to CHS.
Impractical for the clinic?
While capsaicin may be effective, it also can be painful.
LaPoint said that he sometimes sees patients who write on intake forms that they have a capsaicin allergy — either to avoid a burning belly or in hopes of securing stronger painkillers.
“I’ve come across patients who are like, ‘Oh my God, I hate that capsaicin. Whoever thought of that, I’d like to get ahold of them,’” LaPoint said. “I’m like, ‘Bro, it was my fault.’ … They usually laugh.”
Because his hospital in San Diego — “craft beer, craft weed” — sees so many patients with CHS, LaPoint said, he spends some time educating fellow physicians in how to help: “Don’t miss a serious surgical emergency. Don’t blow this off as ‘just cannabis hyperemesis.’ Don’t keep scanning them; and don’t get them started on opioids. Other than that, there’s a lot of room for style.”
While the medical literature is clear that the only way to stop experiencing CHS is to stop using cannabis, LaPoint said, he often counsels patients to use a lower-THC formulation — advice they may be more likely to follow.
When Stuart last saw the defense contractor in Afghanistan, he was in good shape, recovering from days spent vomiting with no ill effects that seemed likely to last. Stuart never did hear what became of the man, who admitted in a roundabout hypothetical fashion to having used the synthetic cannabinoid “spice” to elude drug testing. Stuart said it’s likely, though, that the man’s employer sent him back to the U.S.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

