Using bacteria to clean the environment
In recent years, concerns have heightened about increasing amounts of drugs in the environment, particularly in water. While the impact of this environmental pollution is not well understood, some evidence indicates that these drugs may be entering the food chain. Researchers believe that most of the drugs that end up in fresh water first accumulate at wastewater treatment facilities. Therefore, there is a need to eliminate the drugs at these facilities.
Ashley Robinson, a senior biochemistry major at Hamline University who plans to start graduate school in the fall, started doing research in her sophomore year. She is presenting a poster at the 2021 ASBMB Annual Meeting on this topic, the third research project she has worked on with Betsy Martínez–Vaz.

The researchers’ goal was to find bacteria that break down metformin, a drug commonly used to treat diabetes in the U.S. and around the world. Little research has been done on the impact of pollution with metformin and its byproduct, guanylurea, which are not fully metabolized by humans and thus are excreted into wastewater systems. “We consider them to be emerging pollutants,” Robinson said.
Studies have demonstrated the potential for metformin to disrupt some hormones, she explained. The drug is considered an endocrine disruption agent in some small fishes, and guanylurea has been shown to interfere with the nitrogen cycle in soil. Little is known about its bioaccumulation potential.
“Can these molecules pass up the food chain?” Robinson said. “That is one concern that we have.”
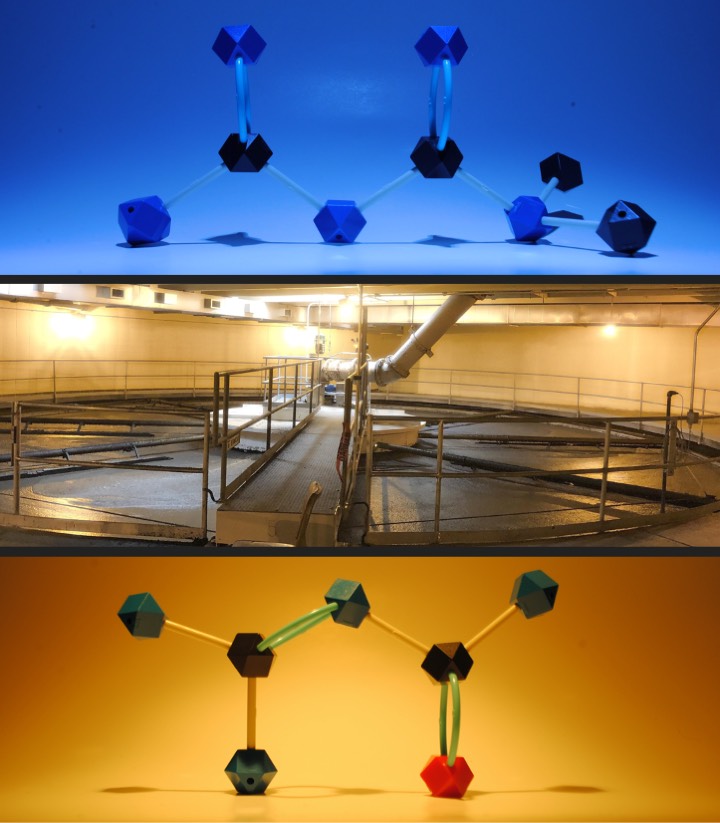
The research team collected samples at a local wastewater treatment facility from several stages of the treatment process. The bacteria in the samples were then grown in the lab under limiting conditions, meaning the bacteria were not given all the nutrients they needed. In this case, their only source of nitrogen was metformin, so most of the bacteria that survived were species that could use metformin as a nitrogen source. The team then used metagenomics to identify the enzymes involved in the breakdown of guanylurea and its transformation product guanidine. They identified three enzymes: guanylurea hydrolase, carboxyguanidine deiminase and allophanate hydrolase.
Robinson and her colleagues are now working to identify the enzyme that breaks down metformin in the initial step that forms guanylurea. They hope the enzymes they find could be used to break down metformin and guanylurea at wastewater treatment facilities, keeping these pollutants out of freshwater systems.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

