Overcoming missed connections to battle Alzheimer’s
A recent study revealed that a protein important for neuron communication is associated with patient resistance to Alzheimer’s disease and may delay cognitive decline.
Like the cord that connects a telephone handset and the receiver, a neuron needs to make a physical connection with other neurons to communicate and transmit signals, which allow people to think and speak. The new study published in the journal Molecular & Cellular Proteomics suggests a protein, called neuritin, may allow some people to retain their neuronal connections even when toxic substances that cause Alzheimer’s attempt to break them down.
Alzheimer’s is the most common cause of dementia and affects more than 5.8 million Americans. To diagnose it, physicians use mental competency tests, physical and neurological exams, brain imaging, spinal fluid tests and medical history. Most patients show both a cognitive decline as well as toxic protein accumulation in the brain, which causes neuron death and brain shrinkage. These abnormal protein aggregates, called amyloid beta plaques and tau tangles, can disrupt neuronal connections and communication, which leads to memory loss and confusion, the hallmark symptoms of Alzheimer’s.
However, some patients show characteristic signs of Alzheimer’s pathology in their brains when examined but remain mentally competent. These individuals are known as “cognitively resilient” by the researchers who conducted the study.
“How cognitively normal older individuals with Alzheimer’s disease pathology withstand dementia onset is one of the most pivotal, unanswered questions in the field,” said Jeremy Herskowitz, associate professor of neurology at the University of Alabama at Birmingham School of Medicine and co-supervisor of the project.
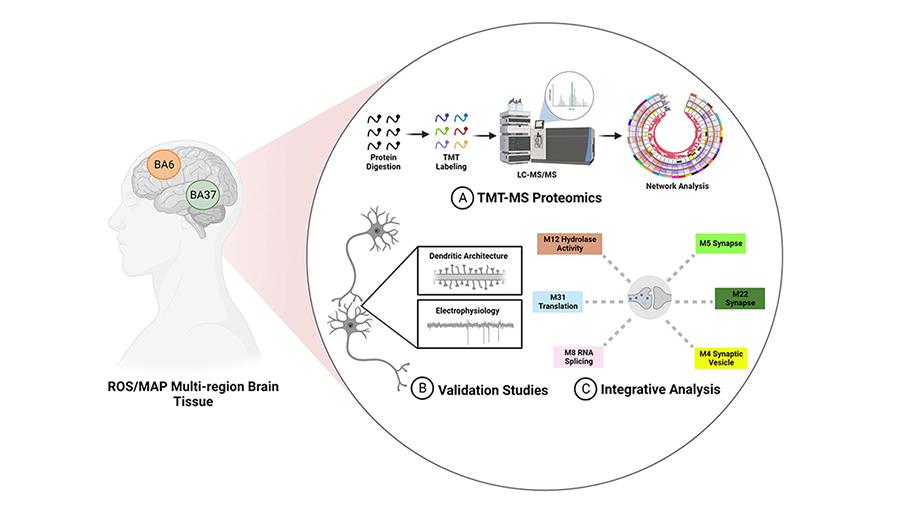
To tackle this question, Herskowitz and Nicholas Seyfried, a professor of biochemistry at Emory University School of Medicine and co-supervisor of the project, teamed up and combined their specialties in proteomics and basic neurology to examine proteins in human brain tissues.
Unlike widely employed hypothesis-driven research, this team studies diseased humans and their tissues first to discover potential therapeutics.
“That's quite different than traditional approaches, which try to make discoveries in experimental model systems,” Herskowitz said. “Our research collectively identifies differences in humans first. Then, after that discovery is made, we can ask questions in experimental model systems to work out what's going on at the molecular and cellular level.”
The researchers conducted a large mass spectrometry screen of the proteins found in the brains of healthy people, typical Alzheimer’s patients and cognitively resilient patients. Cheyenne Hurst, a graduate student at Emory and co-lead author on the study, used high-powered computer programming to determine that neuritin correlates with intact cognitive function over time.
“The higher the amount of neuritin you have in your brain, the more likely you are to be cognitively intact,” Hurst said.
The researchers then wanted to test how the protein affects how neurons communicate. To do this, they isolated neurons from the hippocampus of rats and treated them with either neuritin, the pathogenic amyloid beta or both.
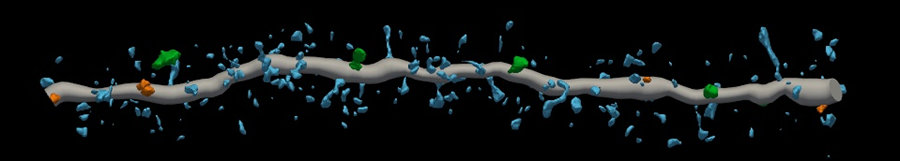
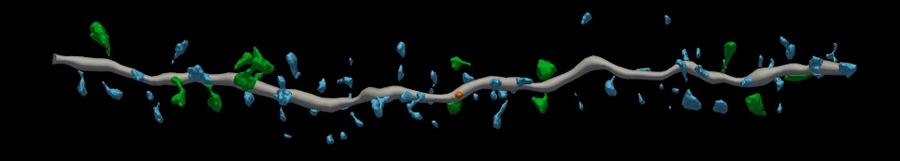
Derian Pugh, a graduate student at UAB and co-lead author, noticed structural differences in the three groups.
“The dendritic spines or synapses coming off healthy neurons kind of remind me of branches on a tree,” Pugh said.
But the structure of the neurons exposed to pathogenic amyloid beta was disrupted — and so were their connections with other neurons. Pugh said they “looked like a tree with no branches.”
However, neuritin completely blocked the detrimental effects of amyloid beta on the neuron cultures.
“With these experiments, we were able to recapitulate what happens in humans that display cognitive resilience and a possible mechanism,” Herskowitz said.
The team plans to focus on the basic biology of neuritin but also on how they can harness neuritin as a biomarker of Alzheimer’s or a therapeutic.
“The ability to estimate the amount of amyloid beta pathology in an older person's brain using biomarkers is getting very advanced,” Seyfried said. “We can predict quite accurately the presence of amyloid beta is in someone's brain while they're still alive. If they have a large amount of amyloid beta, but they're still cognitively normal, they may want to one day get treated with neuritin or drugs that boost neuritin levels so that those symptoms don't develop into dementia.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

