From the journals: JBC
Hijacking chaperones for iron. A new cause of male infertility. Novel regulation of a ribosomal factor. Read about papers on these topics recently published in the Journal of Biological Chemistry.
Hijacking chaperones for iron
Iron is a critical element for pathogen survival, growth and virulence. Many pathogens have developed strategies to obtain iron from hosts, while hosts have evolved to block iron availability to pathogens. Researchers recently have shown that poly(rC)-binding proteins, or PCBPs, act as chaperones to load iron into ferritin, an iron-binding protein, for sequestration; however, pathogenic manipulation of PCBPs remains relatively unexplored.
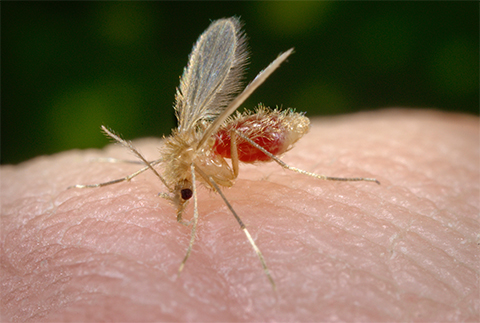
Leishmania donovaniis a parasite that travels between sandfly vectors and vertebrate hosts. In the vertebrate host, Leishmania, which causes the potentially fatal neglected tropical disease visceral leishmaniasis, resides in macrophages and requires iron to maintain metabolic and defensive activities. In a recent article published in the Journal of Biological Chemistry, Sandhya Sen, Saswat Kumar Bal and colleagues at Jawaharlal Nehru University in New Delhi demonstrated that L. donovani is able to cleave PCBPs. Further experiments identified the protease responsible as the secreted zinc-metalloprotease GP63. The authors demonstrated that GP63-mediated cleavage of PCBPs prevents loading of iron onto ferritin and its subsequent sequestration. This hijacking of iron sequestration increases its availability and likely promotes growth and virulence of the pathogen.
This marks the first report of this novel strategy by pathogens to interfere with iron sequestration via ferritin by cleaving chaperone proteins to gain survival advantages within a host.
A new cause of male infertility
Gene associated with retinoid interferon-induced mortality 19 (Grim-19) is best known as an essential component of respiratory complex I in mitochondria, regulating apoptosis and energy metabolism. However, mounting evidence suggests an additional role for Grim-19 in male reproduction.
In a recent study published in the Journal of Biological Chemistry, Hu Qu and colleagues at the Sixth Affiliated Hospital of Sun Yat-Sen University in Guangzhou, China, demonstrated that Grim-19 is expressed in Leydig cells (cells in the extracellular matrix–filled space adjacent to the seminiferous tubules that produce the male sex hormone testosterone) of mice from puberty onward and localized to mitochondria. Using mouse models deficient in Grim-19 expression, the authors showed that loss of Grim-19 led to reduced testosterone production and increased oxidative stress and subsequently to germ cell apoptosis and male infertility, or azoospermia. The authors propose two mechanisms by which Grim-19 could affect fertility: inhibition of cellular signaling via the steroidogenic acute regulatory protein or exacerbation of the inhibitory role of the extracellular matrix on testosterone production via increased integrin activation.
These findings suggest that Grim-19 plays an important role in male sex hormone production and that its modulation could cause or be used to treat testosterone deficiency–related disorders or male infertility.
Novel regulation of a ribosomal factor
Human YVH1, or hYVH1, is a dual-specificity phosphatase that protects cells from environmental stress such as oxidative stress and heat shock, disassembles stress granules containing stalled ribosomes, and promotes biogenesis of the large ribosomal subunit. However, the mechanisms of hYVH1 action and regulation are unclear.
Ashley DaDalt and colleagues at the University of Windsor report in a recent publication in the Journal of Biological Chemistry that a phosphorylation event at Tyr179 on hYVH1 (mediated by the kinase Src) attenuated its localization to stress granules, promoted its translocation to the nucleus and enhanced its association with the 60S ribosomal subunit. Furthermore, the authors performed quantitative proteomics, the analysis of which revealed that Src and hYVH1 coexpression reduced the formation of stalled ribosomal intermediates via downregulation of three cofactors: nucleolin, Y-box binding protein 1 and interferon-related developmental regulator 1. These results were consistent with the hypothesis that phosphorylation of hYVH1 recycles the protein to the nucleus to maintain actively translating ribosomes, reflecting the translational fitness of the cell.
Taken together, these findings provide the first evidence that tyrosine phosphorylation regulates YVH1 activity and suggest that collaborative regulation of YVH1 and Src can maintain a cell’s readiness to combat environmental stressors.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

