COVID-19 vaccines focus on the spike protein, but there’s another target
The latest results from the phase 3 COVID-19 vaccines trials have been very positive. These have shown that vaccinating people with the gene for SARS-CoV-2 spike protein can induce excellent protective immunity.
The spike protein is the focus of most COVID-19 vaccines as it is the part of the virus that enables it to enter our cells. Virus replication only happens inside cells, so blocking entry prevents more virus being made. If a person has antibodies that can recognise the spike protein, this should stop the virus in its tracks.
The three most advanced vaccines (from Oxford/AstraZeneca, Pfizer/BioNTech and Moderna) all work by getting our own cells to make copies of the virus spike protein. The Oxford vaccine achieves this by introducing the spike protein gene via a harmless adenovirus vector. The other two vaccines deliver the spike protein gene directly as mRNA wrapped in a nanoparticle. When our own cells make the spike protein, our immune response will recognise it as foreign and start making antibodies and T cells that specifically target it.
However, the SARS-CoV-2 virus is more complicated than just a spike protein. There are, in fact, four different proteins that form the overall structure of the virus particle: spike, envelope (E), membrane (M) and nucleocapsid (N). In a natural infection, our immune system recognises all of these proteins to varying degrees. So how important are immune responses to these different proteins, and does it matter that the first vaccines will not replicate these?
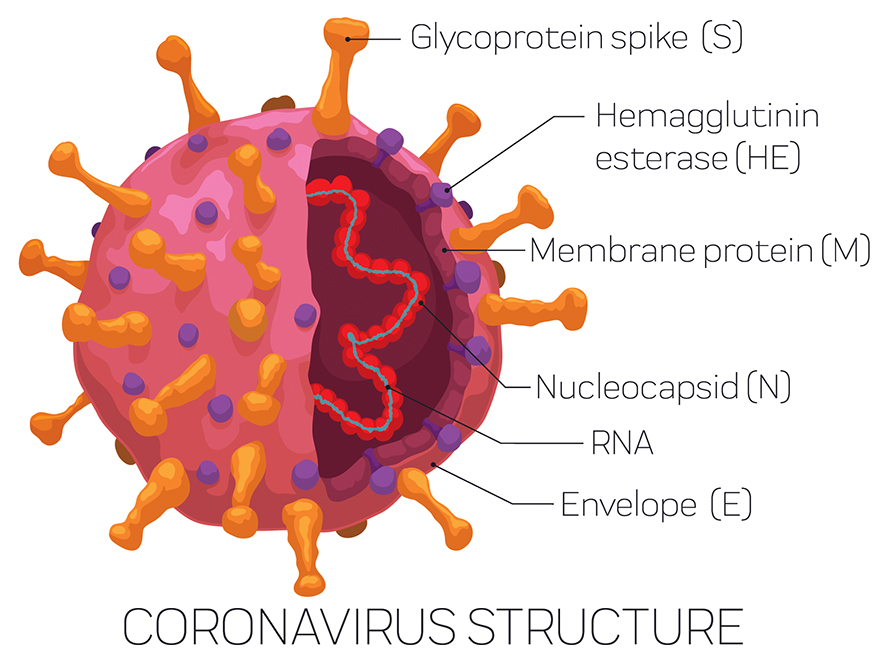
Following SARS-CoV-2 infection, researchers have discovered that we actually make the most antibodies to the N protein – not the spike protein. This is the same for many different viruses that also have N proteins. But how N protein antibodies protect us from infection has been a long-standing mystery. This is because N protein is only found inside the virus particle, wrapped around the RNA. Therefore, N protein antibodies cannot block virus entry, will not be measured in neutralisation assays that test for this in the lab, and so have largely been overlooked.
New mechanism discovered
Our latest work from the MRC Laboratory of Molecular Biology in Cambridge has revealed a new mechanism for how N protein antibodies can protect against viral disease. We have studied another virus containing an N protein called lymphocytic choriomeningitis virus and shown a surprising role for an unusual antibody receptor called TRIM21.
Whereas antibodies are typically thought to only work outside of cells, TRIM21 is only found inside cells. We have shown that N protein antibodies that get inside cells are recognised by TRIM21, which then shreds the associated N protein. Tiny fragments of N protein are then displayed on the surface of infected cells. T cells recognise these fragments, identify cells as infected, then kill the cell and consequently any virus.
We expect that this newly identified role for N protein antibodies in protecting against virus infection is important for SARS-CoV-2, and work is ongoing to explore this further. This suggests that vaccines that induce N protein antibodies, as well as spike antibodies, could be valuable, as they would stimulate another way by which our immune response can eliminate SARS-CoV-2.
Adding N protein to SARS-CoV-2 vaccines could also be useful because N protein is very similar between different coronaviruses – much more so than the spike protein. This means it's possible that a protective immune response against SARS-CoV-2 N protein could also offer some protection against other related coronaviruses, such as Mers.
Another potential benefit that may arise from including N protein in SARS-CoV-2 vaccines is due to the low mutation rates seen in the N protein sequence. Some changes to the sequence of SARS-CoV-2 have been reported over the course of this pandemic, with the most significant changes occurring in the spike protein. There is some concern that if the spike sequence alters too much, then new vaccines will be required. This could be similar to the current need for annual updating of influenza vaccines. However, as the N protein sequence is much more stable than the spike, vaccines that include a component targeting the N protein are likely to be effective for longer.
The first wave of SARS-CoV-2 vaccines brings genuine hope that this virus can be controlled by vaccination. From here it will be an ongoing quest to develop even better vaccines and ones that can remain effective in the face of an evolving virus. Future vaccines will probably focus on more than just the spike protein of SARS-CoV-2, and the N protein is a promising target to add to the current strategies being considered.![]()
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

