Biochemists and molecular biologists sweep major 2025 honors
October was a banner month for biochemistry and molecular biology. Researchers across the field earned major honors, including MacArthur Fellowships, a Nobel Prize and the Kimberly Prize, celebrating discoveries that are transforming medicine and deepening our understanding of life at the molecular level.
MacArthur Fellowship goes to structural biologist

The MacArthur Foundation announced the class of 2025 “Genius Grant” fellows, 25 innovators each awarded $800,000 for creativity, impact and promise. Among them was structural biologist Jason McLellan, professor of molecular biosciences at the University of Texas at Austin.
McLellan uses X-ray crystallography to reveal how viral surface proteins fold, function, and interact. By introducing precise sequence changes, his team can “lock” viral proteins into stable shapes that elicit strong, predictable immune responses. These engineered proteins became the blueprint for groundbreaking vaccines against the respiratory syncytial virus, a pathogen that can cause severe illness in infants and older adults, and SARS-CoV-2, the virus that causes COVID-19.
McLellan’s next goal: a universal coronavirus vaccine to protect against illnesses, including COVID-19, the common cold and more. In a press release, the MacArthur Foundation described McLellan’s work as “critical to protecting human health from the continual emergence of new infectious diseases.”
Nobel Prize honors work on immune tolerance
The Nobel Prize in Physiology or Medicine was awarded to Mary Brunkow of the Institute for Systems Biology in Seattle, Fred Ramsdell of Sonoma Biotherapeutics and Shimon Sakaguchi of Osaka University for uncovering how a class of immune cells calls T regulatory cells provides the molecular basis of immune tolerance, the system that prevents immune cells from attacking the body’s own tissues.
A healthy immune system relies on T cells to recognize and attack foreign invaders. But sometimes, these cells mistakenly target the body’s own tissues, a misfire that triggers autoimmune disease.
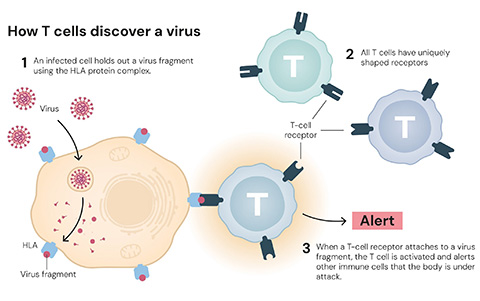
In 1995, Sakaguchi discovered a rare subset of T cells that suppress immune activity called regulatory T cells, or Tregs. His finding reframed the immune system not only as an engine of activation, but as one equally dependent on suppression for balance and self-tolerance.
Around the same time, Brunkow and Ramsdell were studying a mouse line called scurfy, which suffered from an unknown autoimmune disorder. Brunkow and Ramsdell knew the mutation was linked to the X chromosome and combed through the almost 170 million chromosomal base pairs to find the genetic link. They identified Foxp3 as the responsible gene and proposed that it was a transcription factor. They also established a genetic link to human disease, showing that the FOXP3 gene in humans was associated with a rare autoimmune disease called IPEX. Subsequently, Sakaguchi demonstrated that Foxp3 is a master regulator of regulatory T cells and critical for their development.
Their collective work laid the foundation for understanding how the body regulates immune self-tolerance and identified new pathways for treating autoimmune diseases and cancer. In a press release, Olle Kämpe, chair of the Nobel Committee, said “their discoveries have been decisive for our understanding of how the immune system functions.”
Kimberly Prize recognizes biochemist

Biochemist Svetlana Mojsov, a research associate professor at the Rockefeller University, received the Kimberly Prize in Biochemistry and Molecular Genetics from the Northwestern University Feinberg School of Medicine and the Simpson Querrey Institute for Epigenetics. The $250,000 prize, coordinated by Kimberly Querrey in honor of her late husband and Northwestern benefactor Lou Simpson, is presented annually to a scientist whose research into foundational molecular biology has led to clinical advances that improve human health.
Mojsov identified and characterized glucagon-like peptide 1, or GLP-1, a gut hormone that helps regulate blood sugar, appetite and metabolism. When nutrients enter the digestive tract, L cells in the gut release GLP-1, which then signals the pancreas to secrete insulin.
Her discovery paved the way for GLP-1 receptor agonists—a new class of drugs that mimic the hormone’s effects. These medicines, now household names, are transforming the treatment of type 2 diabetes, obesity and metabolic disease.
In a press release, Ali Shilatifard, director of the Simpson Querrey Institute for Epigenetics, said Mojsov’s work “exemplifies the complete arc of discovery to global impact” and has “laid the foundation for life-changing therapies for type 2 diabetes and obesity.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in People
People highlights or most popular articles

Simcox wins SACNAS mentorship award
She was recognized for her sustained excellence in mentorship and was honored at SACNAS’ 2025 National Conference.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Thiam elected to EMBO
He was recognized during the EMBO Members’ Meeting in Heidelberg, Germany, in October.

