The perfect storm
The SARS-CoV-2 pandemic surged around the globe like a vast hurricane, and just a year later some people may have thought the COVID-19 vaccine appeared out of nowhere.
But, hurricanes can take weeks to form in the sea and sky, spurred by multiple factors including angular momentum of the Earth’s rotation, low atmospheric pressure, warm ocean temperatures and thunder.
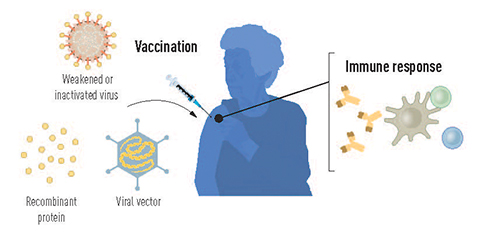
Likewise, the complex technology behind the COVID-19 vaccines was in the works decades prior to their first authorized use in 2020. Katalin Karikó and Drew Weissman developed the foundations for the messenger RNA technology in the late 1990s and early 2000s. Robert Langer conceptualized the lipid nanoparticle carriers even earlier, the 1970s.
Laura Breda, a research assistant professor, and Osheiza Abdulmalik, a research associate, are now working with Weissman, Hamideh Parhiz, a research professor at the University of Pennsylvania School of Medicine, and Stefano Rivella, a professor of pediatrics at the UPenn School of Medicine, to develop a “one-shot” gene therapy cure for sickle cell disease and other hemoglobinopathies using technology similar to that used in the COVID-19 vaccine. Without Karikó and Weissman’s seminal work, Breda and Abdulmalik said, none of their studies would be possible.
The foundation
Hungarian-born Karikó and American Weissman won the 2023 Nobel Prize in physiology or medicine “for their discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19.”
Journal of Biological Chemistry Editor-in-Chief Alex Toker said their discovery “was instrumental in the design of mRNA vaccines and ultimately paved the way for the development of the COVID-19 vaccine therapies that saved millions of lives during the recent pandemic.”

He added: “This underscores the importance of fundamental discoveries in basic mechanisms of cell and molecular biology and their transformative impact on human health.”
Karikó and Weissman’s work was all about demonstrating that an mRNA vaccine must find the “Goldilocks” zone when interacting with the human immune system. Too much recognition of an mRNA vaccine by the immune system could lead to hyperinflammation, elimination of the vaccine material and intense patient symptoms. At the other end of the spectrum, too little mRNA interaction with the immune system and the vaccine will go undetected, failing to induce the required immunity to protect a patient from future disease.
Karikó, a professor at the University of Szeged in Hungary and an adjunct professor at the UPenn School of Medicine, has devoted much of her career to understanding mRNA.
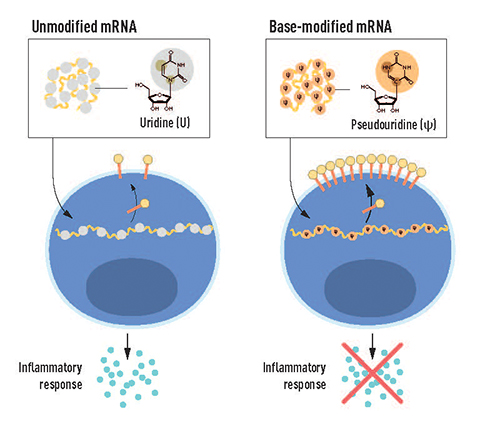
In its natural form in the human body, mRNA consists of four nucleosides: adenine, cytosine, guanine and uridine. Early attempts to use lab-produced mRNA as a vaccine were fraught with problems: It was unstable, difficult to deliver and led to excessive host inflammation.
In the face of these challenges, Karikó teamed up with Weissman, a UPenn professor of vaccine research and immunologist. The two met at the university in the 1990s while using a photocopier, according to a UPenn press release.
Weissman was an expert on dendritic cells and the immune response to vaccines. The two observed that lab-made RNA activated the inflammatory immune response in dendritic cells, while mammalian RNA did not. The differentiating culprit was the chemical makeup of the nucleoside uridine.
In a 2004 JBC paper, Karikó, Weissman and colleagues detailed their finding that mRNA secondary structure activates the immune system to produce proinflammatory cytokines via binding to Toll-like receptor 3, a nucleotide-sensing TLR that is activated by double-stranded RNA, a sign of viral infection or necrotic tissue.
One year later, in a groundbreaking study, the team showed that uridine is recognized by human TLRs on dendritic cells, whereas pseudouridine, a post-transcriptional modified version of uridine, can subvert recognition. mRNA species containing pseudouridine alter the mRNA’s interactions with RNA and proteins such as the enzymes that regulate protein production. Not only did the modified uridine suppress the inflammatory response, but it boosted mRNA-derived protein production as well.
“They were able to transform the in vitro transcribed mRNA into a more stable molecule capable of escaping immune response by just changing its structure,” Breda said. “These changes made it more similar to one of the mRNA species in our body that essentially is not recognized by the immune system. This was not trivial, and it was so advantageous because it opened up a door that no one thought was possible before.”
The pair founded RNARx, a company focused on developing mRNA therapeutics for a wide range of diseases, in 2006. But they struggled to find investors, and the company eventually ran out of funding. In 2013, Karikó joined BioNTech as senior vice president and head of RNA protein replacement to further refine this vaccine technology. Helmut Jeggle, chair of the BioNTech supervisory board, credits Karikó as one of the “pioneering scientists who significantly contributed to the establishment of mRNA as a new drug class.”
Karikó and Weissman’s efforts made possible the first approved mRNA vaccine, today known as the Pfizer-BioNTech COVID-19 vaccine. Karikó remained with the company until 2022.
Wrapping it up
mRNA is inherently fragile and unstable. In cells, it is degraded within days. Karikó and Weissman worked to optimize mRNA stability, but without a delivery mechanism, the mRNA vaccine material, once injected into the body, would be rapidly destroyed without reaching its cellular destination.
mRNA won the Nobel Prize, but many, including Breda, believe the COVID-19 vaccine might not exist without its carrier: the lipid nanoparticle, or LNP, a tiny sphere of fat that remains stable inside the body. Like Karikó and Weissman’s work on mRNA, the field of macromolecule delivery was born almost 50 years before the COVID-19 pandemic.

Robert Langer began laying the foundations for the COVID-19 vaccine at the bench in Judah Folkman’s laboratory as a postdoctoral fellow. At the time, the scientific community believed it was impossible to encapsulate nucleic acids, Langer said. However, in 1976, Langer and Folkman did it using lipophilic polymers, predecessors of today’s LNPs.
Though the work was published in the journal Nature, Langer said that he was widely criticized.
“My first nine grants were rejected, and I could not get an assistant professor position in any chemical engineering department in the world,” Langer said.
Eventually, Langer secured a position at the Massachusetts institute of Technology as an assistant professor of nutritional biochemistry. “Over time, our continued work and that of others changed people’s thinking about what was possible,” he said.
Indeed, Langer said one of the biggest advances in LNPs came with the development of lipid libraries, which allowed scientists to fine-tune the properties of the LNP and its cargo. Over time, LNPs became more diverse and decorated, like a patchwork quilt composed of various threads, shapes, colors and fabrics.
“The earliest of what some people call lipid nanoparticles would probably be considered liposomes,” Langer said. “They were generally composed of lipid bilayers and had an aqueous interior. The current LNPs are more solid. They usually contain cholesterol, a structural lipid, a polyethylene glycol lipid and an ionizable lipid.”
Similar to its mRNA cargo, the LNP had to not stimulate the immune system too much if it were to reach its final destination. Langer credits polyethylene glycol and ionizable lipids with circumventing immune recognition.
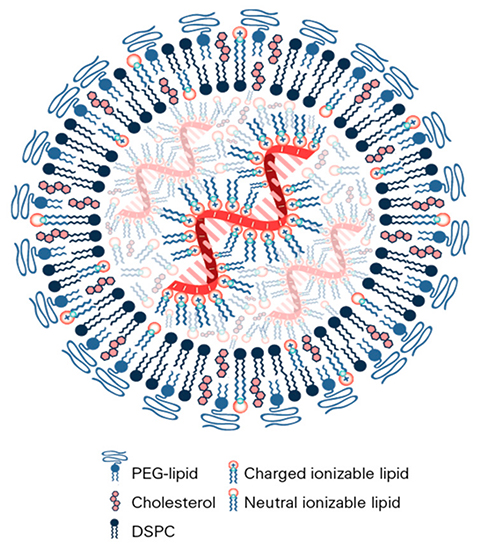
“Ionizable materials are important in that they are neutrally charged at a physiologic pH and positively charged at an acidic pH,” Langer said. “So, they are less toxic than purely positively charged materials.”
Ionizable lipids also allow the LNP to escape the cell endosome, once ingested, and avoid destruction in the lysosome, Langer said. After executing endosomal escape, the LNP delivers its vaccine payload to the cellular cytosol, where proteins such as the SARS-CoV-2 spike can be produced.
This and other work led Langer to co-found Moderna, where he played a key role in rolling out one of the first COVID-19 vaccines. Now a professor at the Massachusetts Institute of Technology, he has authored more than 1,500 scientific papers and is one of the most-cited engineers in history.
LNPs have had some great successes, but there is room for improvement, Langer said. He hopes future LNPs can be targeted to specific tissue and cell types and overcome anatomical roadblocks such as the blood–brain barrier. Weissman and colleagues Rivella, Parhiz, Breda and Abdulmalik have already published a study showing it is possible to target LNPs directly to hematopoietic stem cells to treat disorders such as sickle cell disease.
Beyond the nuts and bolts
Thomas Perlmann, secretary of the 2023 Nobel Committee for Physiology or Medicine, phoned each laureate before making the public announcement in Swedish and English. “They were very happy,” Perlmann said.
He asked the scientists if they were surprised by the announcement because he suspected “they may not be, having received many prizes.” However, both Karikó and Weissman were surprised and overwhelmed by the news, Perlmann said.
This achievement was particularly overwhelming for Karikó, Perlmann said, because she never received a tenured position at UPenn or an R01 grant from the National Institutes of Health, which Perlmann described as a “clear sign that she struggled and didn’t get recognition for the importance of her vision.”
The number of graduate students in science, engineering and health has increased more than 23% since 2017, according to the National Center for Science and Engineering Statistics. However, the number of available tenure-track academic positions is declining. Therefore, more Ph.D. graduates in science and health are looking to fulfill their career aspirations outside of academia, similar to Karikó.
Ayantika Sen is a postdoctoral fellow at Stanford University who studies hyperinflammatory responses during SARS-CoV-2 infection. “Dr. Karikó’s career spanned over decades, shuttling between the industry and academia, which sends an important message that impactful research can be conducted in both places; what matters is passion and collaboration with the right people,” Sen said.
She added: “Accomplished researchers like Katalin Karikó and Jennifer Doudna have set great examples for new-generation female researchers like me, who aspire to gather the best of both worlds and make a long-lasting impact in the world with their research.”
Ann Stock, American Society for Biochemistry and Molecular Biology president and a professor of biochemistry and molecular biology at Rutgers University, said she hopes the prize presentation will encourage current and future students to study biochemistry.
“The awarding of the Nobel prize to two biochemists for the application of pseudouridine to the development of mRNA therapies highlights the relevance of fundamental biochemistry and should be motivational for students, especially medical students, who sometimes lack enthusiasm for learning about the nucleoside and amino acid building blocks of nucleic acids and proteins,” Stock said.
Sen said she was overjoyed to see the hard work of immigrant scientist Karikó highlighted on an international stage.
“On multiple occasions, she faced hardships in pursuing her passion and receiving support from her peers, but she kept going,” Sen said. “I think young female researchers have a new role model to look up to and draw inspiration from.”
Public skepticism
Vaccine skepticism has plagued public discourse and caused many people to pause or forgo getting immunized against SARS-CoV-2. Early in the COVID-19 pandemic, 31.6% of the U.S. population was unsure about receiving a vaccine, and 10.8% stated that they would refuse.
During a Q&A session after the announcement, a reporter asked the Nobel committee representatives if they considered the prize a “powerful strike back for the anti-vaccine movement.”
Olle Kämpe of the Karolinska Institutet, vice chair of the committee, noted that more than 13 billion people have gotten the vaccine and that, while the award would probably not sway those most resistant, "giving a Nobel Prize for this COVID-19 vaccine may make hesitant people take the vaccine and be sure it's very efficient and safe."
Randy Schekman, who received the 2013 Nobel Prize in physiology or medicine for his work on the machinery regulating vesicle traffic, said this prize is a “welcome antidote to the toxic nonsense spread by vaccine skeptics who use misinformation to sow confusion and doubt.”
Future of mRNA vaccines
Gunilla Karlsson–Hedestam, chair of the Nobel committee, emphasized how much this “flexible and fast” vaccine platform has and will continue to impact the general population and cited current research developments on influenza and therapeutic cancer mRNA vaccines.

As for the future of vaccine delivery, Langer said the possibilities are endless, including better lipid platforms, polymers, carbohydrates, micelles, exosomes and hybrids of many components.
Weissman and collaborators recently published a study showcasing a new method of LNP generation using microfluidic chips that will drive more precise and scalable production to meet the stringent manufacturing standards of the pharmaceutical industry.
Craig Martin is a professor of chemistry at the University of Massachusetts Chan Medical School who studies the structure and function in enzyme–nucleic acid interactions. “Karikó and Weissman have launched a field that is just now emerging,” Martin said. “mRNA vaccines are just the beginning. mRNA can deliver therapeutic proteins to cells and offers the promise of personalized cancer therapies. It is truly an exciting time for mRNA."
Scientists at UPenn and other institutions are now looking to adopt the COVID-19 vaccine technology to develop vaccines for other viruses, cancer and more.
Weissman and colleagues recently published a preprint showing that delivery of vascular endothelial growth factor A via nucleoside-modified mRNA encapsulated into LNPs may be a viable treatment option for individuals with acute and chronic liver disease. Likewise, Karikó and colleagues recently published a study showing that an mRNA–LNP vaccine can control human papillomavirus-associated tumors in mice.
“The Nobel recognized that it takes a lot of time and effort for the most rudimentary and basic scientific research to translate into clinical work,” Abdulmalik said. “This is the culmination of many, many years of committed and dedicated work of scientists. It basically took the perfect storm to make sure the technology was ready to fight the pandemic.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

