From the journals: December 2018
We offer a selection of recent papers on a variety of topics from the Journal of Biological Chemistry, the Journal of Lipid Research and Molecular & Cellular Proteomics.
Early screening improves disease outcome
 A rare genetic disorder characterized by abnormal storage of fats in the body can be functionally cured by screening newborns and treating them immediately.ZIVYA/WIKIMEDIA COMMONSCerebrotendinous xanthomatosis, or CTX, is a rare autosomal recessive genetic disorder characterized by abnormal storage of fats in the body. The disease is caused by a mutation in a gene that converts cholesterol to bile acid. Accumulation of fats within the brain can lead to neurological symptoms, and people with this disorder often also suffer from jaundice, tendon inflexibility and progressively brittle bones. Certain populations, including Moroccan Jews and the Israeli Druze community, have higher incidence of CTX. The disease can be functionally cured by early diagnosis and treatment from birth onward. Quality of life and success of treatment are diminished when a diagnosis is delayed.
A rare genetic disorder characterized by abnormal storage of fats in the body can be functionally cured by screening newborns and treating them immediately.ZIVYA/WIKIMEDIA COMMONSCerebrotendinous xanthomatosis, or CTX, is a rare autosomal recessive genetic disorder characterized by abnormal storage of fats in the body. The disease is caused by a mutation in a gene that converts cholesterol to bile acid. Accumulation of fats within the brain can lead to neurological symptoms, and people with this disorder often also suffer from jaundice, tendon inflexibility and progressively brittle bones. Certain populations, including Moroccan Jews and the Israeli Druze community, have higher incidence of CTX. The disease can be functionally cured by early diagnosis and treatment from birth onward. Quality of life and success of treatment are diminished when a diagnosis is delayed.
Researchers led by Tzipora Falik–Zaccai of the Galilee Medical Center conducted a prospective study on dried blood spot, or DBS, samples collected from newborns to detect characteristic CTX-associated markers. They also collected samples high-risk Israeli newborns. Falik–Zaccai and colleagues used a two-tiered approach to identify CTX-positive samples. In the first tier, they used flow-injection tandem mass spectrometry to analyze all DBS samples. This approach detected CTX-positive newborns with 100 percent sensitivity and a low false-positive rate. As the second-tier test, they used liquid chromatography mass spectrometry, which detected CTX-positive samples with 100 percent specificity and 100 percent sensitivity. The team's research, recently published in the Journal of Lipid Research, describes the feasibility of a two-tiered screening process to diagnose CTX in newborns using DBS samples. This type of screening could improve early diagnosis of CTX, thereby ensuring early treatment and improving quality of life.
— Courtney Chandler
Seeking new ways to lower cholesterol
ApoE is a key transporter of extracellular cholesterol in humans. ApoE mimetics such as Ac-hE18-NH2 have undergone clinical trials as cholesterol-lowering agents. Roger White and colleagues at the University of Alabama at Birmingham recently developed new analogs by linking different chemical groups to Ac-hE18-NH2 and investigating their ability to lower cholesterol in mice and macaques. Of the three analogs investigated, one in particular was the most effective at reducing plasma cholesterol in mice that lacked apoE and were fed a standard or high-fat diet. This analog was synthesized by linking a 12-carbon myristic acid chain to Ac-hE18-NH2. This analog also was used to treat macaques that had elevated plasma cholesterol levels. A single administration of the analog was enough to reduce cholesterol in the plasma for up to one week, depending on the dose. Cholesterol in low-density lipoproteins also was reduced significantly after treatment and did not return to baseline until one week after treatment. This work, published in the Journal of Lipid Research, may have implications for developing better cholesterol-lowering therapeutics.
Breaking up a pair of Alzheimer’s troublemakers
During Alzheimer’s disease, the protein amyloid-beta aggregates in the brain into insoluble fibrils and soluble oligomers. Amyloid-beta oligomers bind to membrane-associated prion protein. This binding leads to hallmarks of disease like over-phosphorylation of tau protein and destabilization of dendritic spines. In a paper in the Journal of Biological Chemistry, Nadine Rösener and colleagues at Heinrich-Heine-Universität Düsseldorf characterized the complex containing amyloid-beta and prion protein and showed that a D-enantiomeric peptide, identified in a previous screen of potential drug candidates, could inhibit the formation of this complex.
What determines protein complex stability?
Most of the time, individual proteins perform important functions in our systems as part of a larger complex of multiple proteins. A protein’s function is determined by certain moieties or functional groups that are attached to it after it has been synthesized from RNA. Such additions are called post-translational modifications, or PTMs. The location of such modifications within a protein determines its binding affinity with other proteins and the stability of protein–protein interactions under different conditions. A recent collaborative study by Nikolina Šoštarić and researchers in labs across three countries in Europe has identified certain PTMs that stabilize or destabilize protein complexes purified from budding yeast, or Saccharomyces cerevisiae. They focused on the evolutionarily conserved PTMs at a protein–protein interface and used various computational approaches to determine the effect of these PTMs in regulating protein-protein interactions. Their findings, published in the journal Molecular & Cellular Proteomics, suggest that acetylated lysine residues play a stabilizing role while phosphorylated residues play a destabilizing role in protein complexes. Though this study broadens our understanding of the functional role of PTMs, it remains to be seen how these findings from purified protein complexes repeat within developing cells and extrapolate to other organisms.
If you had a nickel for every UTI
E. coli and other bacteria that cause urinary tract infections sometimes carry the gene encoding the metallophore yersiniabactin, or Ybt. Ybt previously was shown to chelate iron and copper ions. In research published in the Journal of Biological Chemistry, Anne Robinson and colleagues at Washington University School of Medicine showed that Ybt additionally scavenges nickel from the extracellular environment and imports it into nickel-requiring enzymes in the bacterium. Nickel-requiring enzymes like urease have been associated with uropathogenicity, suggesting that this nickel-scavenging pathway may be important for understanding UTIs.
Obesity study connects genes, brains and immune cells
 Brain-derived neurotrophic factor, or BDNF, regulates both emotions and appetite.Jacopo Werther/Protein Data BankResearchers from the Burke Medical Research Institute and Weill Cornell Medical College have connected a genetic risk factor for obesity to an immune cell receptor, uncovering a pathway that may pave the way for more precise treatments for metabolic disorders. Their work was published in the Journal of Biological Chemistry.
Brain-derived neurotrophic factor, or BDNF, regulates both emotions and appetite.Jacopo Werther/Protein Data BankResearchers from the Burke Medical Research Institute and Weill Cornell Medical College have connected a genetic risk factor for obesity to an immune cell receptor, uncovering a pathway that may pave the way for more precise treatments for metabolic disorders. Their work was published in the Journal of Biological Chemistry.
A particular variant in the gene encoding brain-derived neurotrophic factor, or BDNF, causes humans and mice to become extremely obese when fed a high-fat diet. BDNF plays several important roles in the brain, including regulating emotions such as anxiety and aggression as well as appetite. The obesity-associated BDNF variant is very common, present in up to 30 percent of Americans and 70 percent of Asians. Sunghee Cho, a professor of neuroscience at Weill Cornell, wanted to know how fat metabolism worked in the population of people carrying this variant.
Her team found that, in mice carrying the human obesity-associated BDNF mutation, another multifunctional protein was implicated: the receptor CD36. When expressed in taste receptors in the tongue, CD36 is responsible for our love of the flavor of fat. (Knockout mice lacking CD36 do not exhibit their typical preference for fatty foods.) When expressed in monocytes and macrophages, CD36 takes up long-chain fatty acids, which provide energy for these long-lived immune cells.
“(CD36) is heavily involved in lipid metabolism,” Cho said. “When it sees a nutrient, it uptakes it.”
When mice with the obesity-associated BDNF gene variant were treated with an inhibitor of CD36, they did not gain as much weight on a high-fat diet and had less insulin resistance and less inflammation. However, the inhibitor did not affect weight gain in mice with the other BDNF variant, suggesting that CD36 is uniquely important in populations that are genetically obesity-prone.
“The import of the findings is that we can manipulate CD36 based on an individual’s genetic makeup,” Cho said.
— Sasha Mushegian
More than one strategy against cancer
Immunotherapy is a promising strategy against cancer, but immune responses against cancer must be quantified and controlled to avoid dangerous side effects from hyperinflammation. Christian Backes and colleagues at Saarland University wrote in the Journal of Biological Chemistry about developing a time-resolved single-cell assay for monitoring the kinetics and mechanisms of how natural killer cells cause target cell death. They found that a single NK cell could kill cancer cells via different mechanisms, either inducing apoptosis or causing membrane disruption and lysis (necrosis). Because these different types of cell death have different effects on the surrounding tumor microenvironment, this variation should be taken into account to fine-tune NK cell-based immunotherapy.
Synthetic surfactants stick around longer
Pulmonary surfactants are protein–phospholipid mixtures secreted into the alveolar space that reduce surface tension where air and liquid meet. Substitutes have been developed to treat patients who need surfactant supplementation, including preterm infants. A paper in the Journal of Lipid Research describes the differences in metabolism between synthetic and animal-derived exogenous surfactants. Anthony Postle and colleagues at the University of Southampton in the U.K. used mice to compare the metabolism of Curosurf, an animal-derived surfactant used by doctors, with that of CHF5633, a synthetic surfactant in clinical trials. Both were labeled with a carbon isotype-containing tracer and introduced to mice intranasally. The researchers extracted lipids from lung lobes harvested at various time points after introduction, and they dectected the tracer using electrospray ionization mass spectrometry. By tracking the hydrolysis products of each surfactant, Postle and colleagues were able to compare their catabolism profiles. They observed that catabolism of synthetic CHF5633 was delayed compared with that of animal-derived Curosurf, although the pathways used for metabolism were similar. They also observed enhanced recycling of the hydrolysis products of CHF5633 into new phospholipid species. Their findings have implications for how the metabolism of new synthetic pulmonary surfactants will be evaluated during clinical trials.
An alternative pathway in brain mitochondria
The mitochondrial calcium uniporter, or MCU, mediates calcium influx into mitochondria, regulating calcium-dependent processes including mitochondrial respiration. James Hamilton and colleagues at Indiana University School of Medicine showed that the role of this transporter may be different in brain-cell mitochondria than in other cell types. Whereas knocking out MCU in liver, heart and skeletal muscle mitochondria completely inhibited calcium uptake, it only slowed but did not completely inhibit uptake in mitochondria from glial cells. This suggests that another, MCU-independent calcium uptake pathway exists in brain mitochondria and may explain why ischemia-reperfusion injury has brain-specific effects. This research was published in the Journal of Biological Chemistry.
Comparing the content of white and brown fat cells
Fat-producing adipose tissue plays an important role in our bodies by making fats, storing them when they are not needed and metabolizing them to release energy under various conditions. The tissue consists of two kinds of cells: white adipocytes that provide insulation between organs and release energy in times of starvation and brown adipocytes, mainly present in newborns, that produce heat by consuming energy. Recent evidence indicates other systemic functions of these cells, a lot of them regulated by hormones, but details of this regulation are not understood.
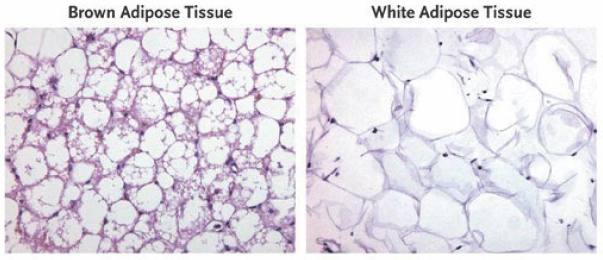 In adipose tissue stained with hematoxylin and eosin, brown adipose tissue is seen to contain multiple lipid droplets (white areas) and mitochondria (purple lining).VAN MARKEN LICHTENBELT ET AL/DIAPEDIA Recent multidisciplinary work by Asrar Ali Khan and researchers at several institutes in Germany, published in the journal Molecular & Cellular Proteomics, compares the secretome of mouse-derived white and brown adipocytes with or without hormonal stimulation. According to this study, while unstimulated brown adipocytes secrete a variety of proteins, unstimulated white adipocytes mainly secrete carbohydrate metabolism-regulating proteins. When the authors stimulated these cells with the hormone norepinephrine (essential for many physiological functions), they found that the brown adipocytes released many novel cytokines (cell-signaling proteins), while white adipocytes secreted more known cytokines. Interestingly, brown adipocytes showed a marked change in their secretome upon hormonal activation.
In adipose tissue stained with hematoxylin and eosin, brown adipose tissue is seen to contain multiple lipid droplets (white areas) and mitochondria (purple lining).VAN MARKEN LICHTENBELT ET AL/DIAPEDIA Recent multidisciplinary work by Asrar Ali Khan and researchers at several institutes in Germany, published in the journal Molecular & Cellular Proteomics, compares the secretome of mouse-derived white and brown adipocytes with or without hormonal stimulation. According to this study, while unstimulated brown adipocytes secrete a variety of proteins, unstimulated white adipocytes mainly secrete carbohydrate metabolism-regulating proteins. When the authors stimulated these cells with the hormone norepinephrine (essential for many physiological functions), they found that the brown adipocytes released many novel cytokines (cell-signaling proteins), while white adipocytes secreted more known cytokines. Interestingly, brown adipocytes showed a marked change in their secretome upon hormonal activation.
Overall, norepinephrine stimulation triggered the adipocytes to secrete a diverse range of proteins regulating various processes apart from carbohydrate metabolism, namely lipid metabolism and adipogenesis. Increased secretion of proteins conferring resistance to oxidative stress also was observed.
This study sheds some light on the response of fat-processing cells to hormonal regulation. Moreover, brown adipose tissue has been identified as a target for the treatment and prevention of obesity and obesity-associated diseases such as Type 2 diabetes. This study provides an archive of the potential biomarkers of activated brown adipocytes, which could be exploited for treating brown adipocyte-associated diseases.
— Isha Dey
Chaos and flexibility in elastin
Elastins are the proteins that allow lungs, arteries and skin to stretch and bend. To understand these essential proteins’ mechanical properties and resilience, we need to know how the network of elastin fibers is cross-linked. In research published in the Journal of Biological Chemistry, Christoph Schräder and colleagues at Martin Luther University Halle-Wittenberg characterized these cross-links in detail in mature bovine elastin. Contrary to the previous assumption that these proteins are assembled in an orderly array, Schräder and colleagues showed that elastin formed an unordered, randomly cross-linked network.
Of dual-coding genes: database revision needed
A gene is a piece of DNA that codes for a protein. It contains a nucleotide sequence called the open reading frame, or ORF, which the RNA polymerase reads and then translates the codons (sets of three bases coding for one amino acid) into messenger RNA, which ultimately forms the protein after some post-transcriptional processing. According to the one gene–one protein hypothesis of George Beadle and Edward Tatum, each gene codes for a single protein. However, a recent paper in the journal Molecular & Cellular Proteomics by Xavier Roucou and researchers from the Université de Sherbrooke in Canada in collaboration with INSERM (Institut National de la Santé et de la Recherche Médicale) in France reiterates the existence of dual-coding genes, that is, single genes coding for different proteins from different ORFs. They showed that the gene MIEF1, known to encode 463-amino-acid protein MiD51 (an important regulator of mitochondrial division), also encodes the 70-amino-acid protein altMiD51. The smaller protein is coded by an exon originally considered noncoding. In fact, in both cell lines and tissues, the smaller protein was expressed two to six times more than the bigger protein. Although expression does not correlate with function, this study means that alternate ORFs must be incorporated in a gene’s coding sequence in the database.
Mitochondrial proteins take a hit in a mouse model of fatty liver
 Kwangwon Lee and a team of researchers at Northeast Ohio Medical University studied the lifespan of mitochondrial proteins in a mouse model of fatty liver disease. Comparing the amount of protein between healthy mice and a mouse model of nonalcoholic fatty liver disease gave them an estimate of each protein’s half-life.
Kwangwon Lee and a team of researchers at Northeast Ohio Medical University studied the lifespan of mitochondrial proteins in a mouse model of fatty liver disease. Comparing the amount of protein between healthy mice and a mouse model of nonalcoholic fatty liver disease gave them an estimate of each protein’s half-life.
Their findings, published in the journal Molecular & Cellular Proteomics, show that many proteins involved in mitochondrial function, especially those directly involved in making ATP, are broken down more quickly than usual in a fatty liver. Not only does this reduce the number of proteins, but the remaining proteins are also less active.
The insult to ATP-producing proteins damaged the mitochondria. In an apparent effort to get rid of dysfunctional mitochondria, cells from fatty livers showed more evidence of digesting their mitochondria but did not increase production of new ones. As a result, the authors observed mitochondrial and ATP shortages in the cells of mice with fatty liver.
The authors proposed that because the overloaded liver cells used fatty acids instead of glucose to make energy, they might have created more reactive oxygen byproducts, which damaged proteins.
— Laurel Oldach
Understanding oddball interferons
The 16 human type I interferons, or IFNs, regulate innate and adaptive immunity. Two of them, IFN-epsilon and IFN-kappa, have very different sequences from the others and are expressed selectively in skin, mucosa and the female reproductive system. In research published in the Journal of Biological Chemistry, Bethany Harris and colleagues at the University of Alabama report biophysical characterizations of these two IFNs’ binding interactions. They found that the two had thousandfold lower potency in signaling through the cell-surface receptor IFNAR2 than other IFNs but also had much lower affinity for an IFN-blocking viral protein. The authors speculate that these features are important for protection against viruses, including HIV, in the reproductive tract.
Stress and iron break ribosomes
Under oxidative stress, ribosomal RNA can break. The mechanism of this breakage was incompletely understood. In research published in the Journal of Biological Chemistry, Jessica Zinskie and colleagues at Rowan University showed that oxidant-induced cleavage of rRNA was site-specific and mediated by cellular iron. When free iron bound to ribosomes at specific sites, its redox activity resulted in rRNA fragmentation. The authors speculate this effect could be an adaptive mechanism for fine-tuning ribosome activity under stress.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.



.jpg?lang=en-US&width=300&height=300&ext=.jpg)
