At the intersection of genetics and neurological health
Many genetic diseases are caused by mutations to the coding regions of DNA that result in production of a truncated protein, which cannot be properly read by target tissues in the body. This shortage of necessary protein supply often cannot be compensated for, and that is the case for spinal muscular atrophy.
SMA is a rare disorder induced by a single mutation of a survival motor neuron gene. The genes SMN1 and SMN2 are responsible for the production of survival motor neuron proteins, which are key in the development of motor function.

Some of the characteristic symptoms of SMA include muscle weakness, limited motility and respiratory complications. It is most often diagnosed using a combination of genetic testing, electromyography and biopsy.
SMA affects approximately one in every 10,000 children born each year.
August is Spinal Muscular Atrophy Awareness Month. In observance, this article covers the genetics, the five subtypes — a prenatal one, three pediatric ones and an adult-onset one — and the existing therapies for the condition.
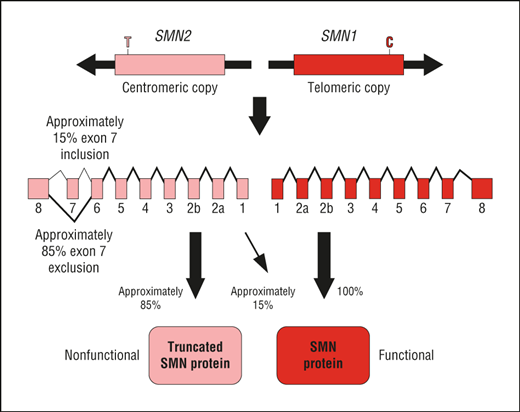
The genetics
SMA was first documented by Guido Werdnig and Johan Hoffman in the 1890s, when they were presented with cases of infants experiencing severe, progressive muscle weakness that persisted throughout the first several months of life. However, it would be almost a century before the origin of the disease could be properly identified.
We now know that SMA is an autosomal recessive condition, meaning that two copies of the mutated gene need to be present for expression of a diseased phenotype.
Approximately one in every 50 Americans is a carrier for the disease.
SMA Types 1–3: Infantile onset
Mutation of the SMN1 gene is the most common cause of spinal muscular atrophy. Typically, a homozygous deletion of exon 7, exon 8, or both, in the SMN1 gene results in SMA type 1. Also referred to as Werdnig–Hoffman disease, SMA type 1 can be identified during infancy. It includes warning signs such as diminished reflexes, hypotonia and respiratory impairment, all of which lead to a drastically shortened life expectancy of only two years.
In addition to complete exon deletion, however, a type of homologous recombination known as gene conversion can also occur in SMN1. In this process, the SMN1 gene is converted into an SMN2 gene. SMN2 plays a similar role to its paralog in the sense that it produces SMN proteins; however, the amount of protein produced by SMN2 is significantly lower than that produced by SMN1. This leads to deficiency, resulting in SMA type 2 or SMA type 3. The amount of SMN2 genes present is inversely correlated to disease severity.
SMA type 2 typically can be diagnosed within six to 18 months of age. It is the second most severe type of SMA, and children who have the condition experience limited motility; patients are able to sit upright on their own but require assistance to properly stand or walk. Additionally, it is not uncommon for those with SMA type 2 to experience respiratory difficulty. These children experience a decreased life expectancy of about 20 to 40 years.
SMA type 3 is the least severe pediatric-onset form of the disease. It is frequently referred to as Kugelberg–Welander disease and can be identified after 18 months of age. These children can walk and sit on their own but may experience difficulty with more complex movements such as climbing. With treatment, children diagnosed with SMA type 3 have an average life expectancy.
SMA Types 0 and 4: Antenatal and adult onset
SMA type 0, also referred to as very severe or antenatal SMA, occurs during fetal development. Similar to SMA type 1, it occurs due to a homozygous deletion of exons 7 and 8. Physicians observe decreased fetal movement in utero, hypotonia and severe respiratory distress in these patients, who have the lowest life expectancy and rarely live beyond six months.
SMA Type 4 is the only adult-onset type. It typically occurs after 21 years of age, and is considered the least severe form due to the high amount of SMN2 copies associated with the condition. Like SMA type 3, individuals with this condition experience an average life expectancy. Additionally, symptom presentation is minimal, with slow progression and some reported loss of ambulation within 20 years of onset.
Current therapeutic approaches
There are two current approaches to therapeutic development for SMA, with an overall goal of achieving either modified splicing of exon 7 or increased transcription of SMN2.
An alteration of exon 7 splicing is meant to increase the total amount of functional SMN1 copies producing integral SMN proteins, while other therapeutics focus on increasing production of these proteins through simply increasing the number of functional SMN2 copies.
Drugs currently in use include:
-
Nursinergen: This antisense oligonucleotide promotes inclusion of exon 7 and production of full-length SMN2 genes. Nursinergen is available for use in patients with all types of SMA but requires tumultuous intrathecal injections and has a high price tag.

- Risdiplam: Approved by the Food and Drug Administration in 2020, this small-molecule drug is the first orally administered SMA therapeutic. Risdiplam increases production of full-length SMN2 copies while simultaneously decreasing the amount of SMN2 copies lacking exon 7. It is available for use in patients of all ages with SMA, making it a seemingly inclusive and more accessible treatment option.
- Zolgensma: Zolgensma is an adeno-associated vector-9 gene-replacement therapy that delivers a functional SMN1 copy to the human genome. This treatment is enticing due to its minimal one-time intravenous injection requirement. However, some research suggests the potential for toxic gain-of-function mutations to occur as a long-term side effect.
Access to medication is one of the most commonly discussed problems in the healthcare world. There are many barriers associated with receiving quality therapeutic treatment, including often-exorbitant costs.
Ringing up at $2.1 million per patient, Zolgensma in 2021 became the most expensive drug in Europe and the priciest treatment to ever be approved by the National Institute for Health and Care Excellence in England.
Because of the price, which is steep due to manufacturing expenses, Zolgensma may not be covered even for insured patients in some cases, making it a promising yet highly inaccessible therapeutic route.
iPSCs for disease modeling
A lot of science has to be done before a drug can enter clinical trials and eventually make it to market. And to lay that groundwork, researchers first need good models for the disease. Some researchers are using stem cells for this purpose.
Known for their regenerative capacity and ability to differentiate into practically any type of cell, stem cells serve as a noninvasive method through which researchers can better characterize disease manifestation in patients.
Induced pluripotent stem cells — a type of stem cell that has been reverted back to an embryonic state — were used as the basis of a recent study by Stanford University scientists.
By taking cells from SMA patients and reverting them into iPSCs, the researchers were able to observe disease progression in real time. Furthermore, iPSC observation allows for completion of in vivo testing prior to clinical trial, thereby providing scientists with a stronger comprehension of how different therapeutics will interact with patients once administered.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

