Modern plant enzyme partners with surprisingly ancient protein
Scientists from the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory have discovered that a protein responsible for the synthesis of a key plant material evolved much earlier than suspected. This new research explored the origin and evolution of the biochemical machinery that builds lignin, a structural component of plant cell walls with significant impacts on the clean energy industry.
When the first land plants emerged from aquatic environments, they needed to adapt in order to survive.
Chang-Jun Liu, a senior scientist in Brookhaven’s Biology Department, said, “The emergence of lignin, which provides structural support for the plants, was a key evolutionary event that enabled plant survival in the new terrestrial environment.”

Understanding how plants developed protective mechanisms that enable survival in new environments is vital as they face challenges imposed by climate change today. But lignin is also of great interest to researchers searching for clean energy options. This tough plant material can be processed and converted into valuable bioproducts. And lignin is the only renewable source of aromatic compounds, which are chemically similar to molecules found in conventional jet fuel and can be used as “drop-in” fuel by airlines.
“Modern plants contain three types of lignin, but most early lignin-containing plants had only two types. The ‘newer’ lignin is called syringyl-lignin, or S-lignin,” explained Liu. S-lignin evolved relatively recently with flowering plants and is structurally less complex than the other lignin components. Its potential industrial applications, in particular, have captured the attention of scientists because S-lignin is relatively easy to break down to simple aromatics.
The new study, recently published in The Plant Cell, builds on years of research focused on lignin and the molecules responsible for its synthesis. In 2019, Liu and his colleagues discovered that a specific cytochrome b5 protein, CB5D, is indispensable for the production of S-lignin but not the other, more ancient types of lignin.
“The uniqueness of CB5D’s role in S-lignin synthesis intrigued us,” Liu noted. “So, we were inspired to further explore its origin and evolution.”

Enzymatic teamwork
In a previous study, Liu’s team found that CB5D has a special partnership with an enzyme called ferulate 5-hydroxylase (F5H). Together, these molecules synthesized the valuable S-lignin.
The scientists knew that the evolution of F5H in flowering plants had led to the production of S-lignin. So, they expected to find that CB5D had co-evolved with F5H.
To explore their hypothesis, the scientists ran a genetic analysis to find other plant species whose DNA contained genes similar to the modern CB5D gene, which acts as instructions for assembling the CB5D protein. They identified 21 species, ranging from evolutionarily ancient to evolutionarily recent. The scientists then synthesized these genes and individually expressed them in a modern plant species that was genetically altered to lack the CB5D gene.
“Without the CB5D gene, the plant synthesizes only a small amount of S-lignin,” said Xianhai Zhao, a postdoctoral researcher at Brookhaven and lead author on the new paper. “But if this function was restored with the expression of one of the related genes, then we would know that gene functions similarly to the modern CB5D gene.”
The scientists discovered that a gene from a green algae species that evolved into an early land plant over 500 million years ago restored S-lignin synthesis in the modern plant. This indicated that the gene exhibited CB5D-type functionality. The scientists also found that the function was conserved in several early land plants, like liverworts and mosses.
“This means that the CB5D evolved millions of years earlier than we had expected,” explained Liu. “It was quite surprising to find that a modern electron acceptor like F5H had partnered with an ancient protein to develop new biochemical machinery that synthesizes the advanced lignin structure.”
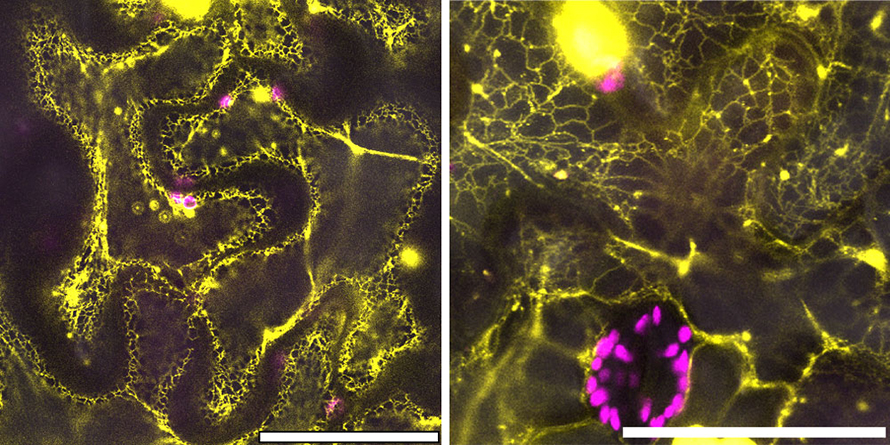
Scientific teamwork and next steps
The CB5Dgene and its more ancient counterpart contained similar DNA sequences and functions. But the scientists wanted to make sure that the CB5D protein from an ancient species, like liverwort, was expressed in the same subcellular structures as modern CB5D.
So, they used confocal microscopy at the Center for Functional Nanomaterials, a DOE Office of Science user facility at Brookhaven Lab, to confirm that this was the case.
Having found ancient genes that encode proteins similar to the modern CB5D protein in terms of S-lignin synthesis in modern plants and cellular localization, the team wanted to learn more about this protein’s ancient function and how it changed or expanded over time.
Their analysis showed the CB5D-like protein emerged in aquatic algae just before they transitioned to a terrestrial environment. And because it was conserved in early land plants, this protein likely serves one or more essential functions.
“Ancient plants like liverwort didn’t contain S-lignin,” said Zhao. “If the CB5D-type protein wasn’t responsible for synthesizing S-lignin, what did it do?”
Liu remarked, “That’s the beauty of research. Answering one question leads you to even more interesting questions waiting to be explored.”
This article is republished from the Brookhaven National Laboratory website, Read the original here.Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

