Zinc is a metal essential to life
All living things, including people, need zinc in their diets. Getting too little of this essential metal can impair growth and cause immune dysfunction, neurological disorders and cancer. Unfortunately, over 17% of the world’s population is at risk for zinc deficiency. The World Health Organization considers this kind of micronutrient-related malnutrition a leading contributor to disease and death.
After you eat a meal, zinc is taken up by the cells of your body. Inside each cell, zinc binds to proteins to support their structure and function. Researchers estimate that up to 10% of all proteins need zinc to properly function. In this sense, a zinc protein without zinc is similar to a car without an engine or without screws holding it together: It either might not work or disassemble completely.
Despite zinc’s importance to human health, several aspects of how it supports cellular processes aren’t completely understood, including how it’s incorporated into the proteins essential for cell function in the first place.
As researchers who study how metals work in biological systems such as the human body, we wanted to understand how zinc is distributed within a cell. Which proteins in the cell get zinc first, especially if there isn’t enough to go around? How does zinc get to these important proteins?
With our colleagues in the Skaar Lab at Vanderbilt University Medical Center and the Giedroc Lab at Indiana University, we recently identified the first known molecule that delivers zinc to crucial proteins.
Delivering zinc to where it needs to go
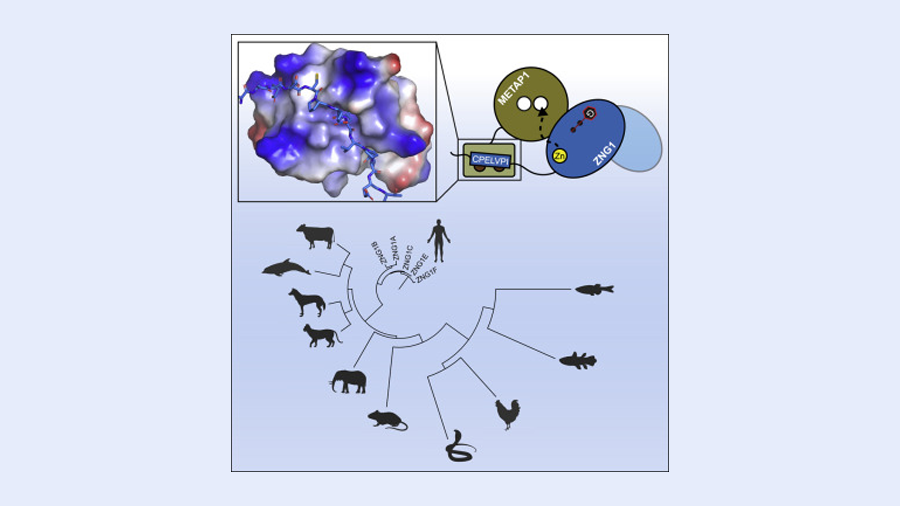
We started by investigating the molecules a cell produces when zinc levels are low. One family of proteins seemed particularly interesting because it looked as if it could be a potential metallochaperone, a protein that selectively inserts metals, such as zinc and iron, into other proteins. We named this protein family ZNG1.
As it turns out, all vertebrates have the gene that directs cells to produce ZNG1. While ZNG1 interacts with several proteins that bind zinc, one in particular, a protein called METAP1, caught our attention. METAP1 is known to activate many other essential proteins within the cell. Cells without functioning METAP proteins cannot survive.
We were intrigued by METAP1 because it interacts with ZNG1 proteins across species – among them zebrafish, mice and people. The finding suggests that the connection between these two proteins has been maintained for over 400 million years of evolution, meaning that the ZNG1’s supportive role in METAP1 function is important in all organisms that produce these proteins.
To study the role ZNG1 plays in animal health, we mutated the gene coding for ZNG1 in mice and zebrafish. When animals without ZNG1 were deprived of zinc, they either failed to grow or displayed developmental defects. Although the animals still have trace amounts of zinc available, they were unable to use the zinc correctly. This confirmed that ZNG1 helps METAP1 function properly, likely by helping it bind to or use zinc.
Using molecular imaging and other methods, we also observed that the energy-producing mitochondria of zinc-starved mouse cells without working ZNG1 proteins were not functioning correctly. This highlights ZNG1’s importance during periods of zinc deficiency by helping the cell allocate trace levels of this essential metal to the mitochondria and ultimately sustain cellular energy production.
ZNG1 may hold the key to zinc deficiency
We believe this research is just the first step to better understand how zinc metallochaperones maintain health and cellular function when zinc levels are low.
We hypothesize that ZNG1 supports the function of additional zinc-dependent proteins in the cell. In that way, ZNG1 would be the gatekeeper that distributes zinc to a network of essential proteins, ultimately allowing an organism to survive even if dietary zinc is limited.
This research paves the way to understanding how cells use zinc during periods of malnourishment or zinc deficiency. Further research on the proteins to which ZNG1 preferentially gives zinc when there isn’t enough available could help identify which cellular processes are most crucial to sustain life when zinc is limited. This in turn could help in the fight against the negative health consequences of zinc deficiency.![]()
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.


