Fun facts about bones: More than just scaffolding
Bones: They hold us upright, protect our innards, allow us to move our limbs and generally keep us from collapsing into a fleshy puddle on the floor. When we’re young, they grow with us and easily heal from playground fractures. When we’re old, they tend to weaken, and may break after a fall or even require mechanical replacement.
If that structural role was all that bones did for us, it would be plenty.
But it’s not. Our bones also provide a handy storage site for calcium and phosphorus, minerals essential for nerves and cells to work properly. And each day their spongy interior, the marrow, churns out hundreds of billions of blood cells — which carry oxygen, fight infections and clot the blood in wounds — as well as other cells that make up cartilage and fat.
Even that’s not all they do. Over the past couple of decades, scientists have discovered that bones are participants in complex chemical conversations with other parts of the body, including the kidneys and the brain; fat and muscle tissue; and even the microbes in our bellies.
It’s as if you suddenly found out that the studs and rafters in your house were communicating with your toaster.
Scientists are still deciphering all the ways that bone cells can signal other organs, and how they interpret and respond to molecular messages coming from elsewhere. Already, physician-scientists are starting to consider how they might take advantage of these cellular conversations to develop new treatments to protect or strengthen bone.
“It’s a whole new area of exploration,” says Laura McCabe, a physiologist at Michigan State University in East Lansing. The recent work has convinced scientists that bone is far more dynamic than once thought, McCabe says — or, as a student of hers used to say, “Bone is not stone.”
Early evidence that bone has something to say
Bone is a unique tissue: It contains not only cells that build the hard matrix that gives the skeleton its strength, but also cells that break it down — enabling bone to reshape itself as a child grows, and to repair itself throughout life. The bone builders are called osteoblasts, and the disassembly crew consists of cells known as osteoclasts. When the balance between the actions of the two is off-kilter, the result is too little (or too much) bone. This happens, for example, in osteoporosis, a common condition of weak and brittle bones that results when bone synthesis fails to keep up with degradation of old bone.
In addition to osteoblasts and osteoclasts, bone contains another cell type, the osteocytes. While these cells comprise 90 percent or more of bone cells, they weren’t studied much until about 20 years ago, when a cell biologist named Lynda Bonewald got interested. Colleagues told her not to waste her time, suggesting that osteocyctes probably only played some mundane role like sensing mechanical forces to regulate bone remodeling. Or maybe they were just kind of there, not doing much of anything.
Bonewald, now at Indiana University in Indianapolis, decided to investigate them anyway. Osteocytes do, in fact, sense mechanical load, as she and other researchers have found. But as Bonewald says, “They do so much more.” She recently wrote about the importance of osteocytes to the kidneys, pancreas and muscles in the Annual Review of Physiology.
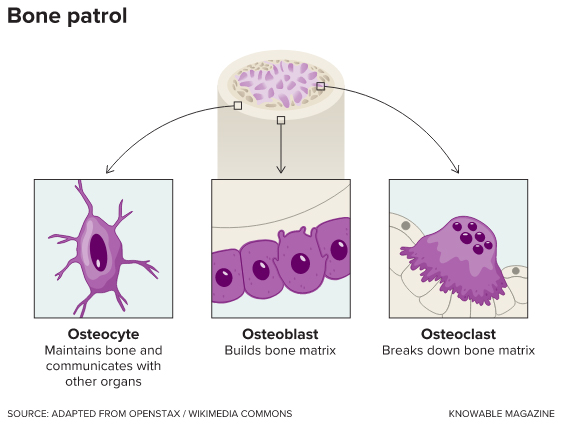
in response to the body’s needs and resources.
Her first finding regarding osteocyte communication with other organs, reported in 2006, was that the cells make a growth factor called FGF23. This molecule then cruises the bloodstream to the kidneys. If the body has too much FGF23 — as happens in an inherited form of rickets — the kidneys release too much phosphorus into urine, and the body starts to run out of the essential mineral. The resulting symptoms include softened bones, weak or stiff muscles, and dental problems.
Around the same time that Bonewald was diving into osteocyte research, physiologist Gerard Karsenty began investigating a potential relationship between bone remodeling and energy metabolism. Karsenty, now at Columbia University in New York, suspected that the two would be related, because destroying and re-creating bone is an energy-intensive process.
In a 2000 study, Karsenty investigated whether a hormone called leptin could be a link between these two biological processes. Leptin is produced by fat cells and is best known as a depressor of appetite. It also emerged in evolution around the same time as bone. In experiments with mice, Karsenty found that leptin’s effects in the brain put the brakes on bone remodeling.
Using leptin in this way, Karsenty suggests, would have allowed the earliest bony creatures to suppress bone growth alongside appetite when food was scarce, saving their energy for day-to-day functions.
His group found support for this idea when they took X-rays of the hand and wrist bones of several children who lack fat cells, and thus leptin, due to a genetic mutation. In every case, radiologists unfamiliar with the people’s true ages ranked the bones as months or years older than they were. Without leptin, their bones had sped ahead, acquiring characteristics like higher density that are more typical of older bones.
That was a case of bone listening to other organs, but in 2007, Karsenty proposed that bone also has something to say about how the body uses energy. He found that mice lacking a bone-made protein called osteocalcin had trouble regulating their blood sugar levels.
In further research, Karsenty discovered that osteocalcin also promotes male fertility via its effects on sex hormone production, improves learning and memory by altering neurotransmitter levels in the brain, and boosts muscle function during exercise. He described these messages, and other conversations that bone participates in, in the Annual Review of Physiology in 2012.
It’s a spectacular set of functions for one molecule to handle, and Karsenty thinks they’re all linked to a stress response that early vertebrates — animals with backbones — evolved for survival. “Bone may be an organ defining a physiology of danger,” he says.
Karsenty proposes that osteocalcin’s effects allowed early vertebrates, both male and female, to respond to the sight of a predator by amping up energy levels, through the effects of testosterone, as well as muscle function. They’d be able to run away, and later remember (and avoid) the place where they’d encountered that threat.
Researchers in Karsenty’s lab did these studies with genetically modified osteocalcin-deficient mice that he developed, and several labs have replicated his results in various ways. However, labs in the US and in Japan, working with different strains of mice that don’t make osteocalcin, didn’t see the same widespread effects on fertility, sugar processing or muscle mass. The scientists haven’t yet been able to explain the disparities, and the danger-response hypothesis remains somewhat controversial.
Whether or not osteocalcin played the big role in vertebrate evolution that Karsenty proposes, these studies have inspired other scientists to examine all kinds of ways that bone listens to and talks to the rest of the body.
Crosstalk between muscle and bone
Bone and muscle, partners in movement, have long been known to interact physically. Muscles tug on bone, and as muscles get stronger and larger, bone responds to this increased physical pull by becoming bigger and stronger too. That allows bone to adapt to an animal’s physical needs, so the proportional muscle and bone can continue to work together effectively.
But it turns out that there’s also a chemical conversation going on. For example, skeletal muscle cells make a protein called myostatin that keeps them from growing too large. In experiments with rodents, alongside observations of people, researchers have found that myostatin also keeps bone mass in check.
During exercise, muscles also make a molecule called beta-aminoisobutyric acid (BAIBA) that influences fat and insulin responses to the increased energy use. Bonewald has found that BAIBA protects osteocytes from dangerous byproducts of cellular metabolism called reactive oxygen species. In young mice that were immobilized — which normally causes atrophy of bone and muscle — providing extra BAIBA kept both bones and muscle healthy.
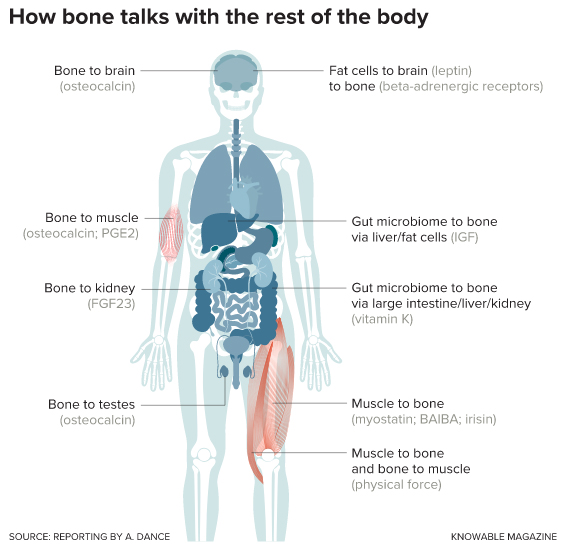
of the relevant messengers are shown in parentheses.
In additional studies, Bonewald and colleagues found that another muscle molecule that increases with exercise, irisin, also helps osteocytes to stay alive in culture and promotes bone remodeling in intact animals.
The conversation isn’t all one-way, either. In return, osteocytes make prostaglandin E2, which promotes muscle growth, on a regular basis. They boost production of this molecular messenger when they experience an increase in the tug from working muscles.
What bone gets from the gut
The human body contains about as many microbial cells as human ones, and the trillions of bacteria and other microorganisms inhabiting the gut — its microbiome — function almost like another organ. They help to digest food and prevent bad bacteria from taking hold — and they talk to other organs, including bone.
So far, the bone-microbiome conversation seems to be one-way; no one has observed bone sending messages back to the microbes, says Christopher Hernandez, a biomechanics expert at Cornell University in Ithaca, New York. But the skeleton can learn a lot of useful things from the gut, McCabe says. For example, suppose a person gets a nasty case of food poisoning. They need all their resources to fight off the infection. “It’s not the time to build bone,” says McCabe.
The first hints of a bone-microbiome connection came from a 2012 study of mice raised in a sterile environment, without any microbes at all. These animals had fewer bone-destroying osteoclasts, and thus higher bone mass. Giving the mice a full complement of gut microbes restored bone mass to normal, in the short term.
But the long-term effects were a bit different. The microbes released molecules called short-chain fatty acids that caused the liver and fat cells to make more of a growth factor called IGF-1, which promoted bone growth.
Gut microbes also appear to moderate another signal that affects bone: parathyroid hormone (PTH), from the parathyroid glands at the base of the neck. PTH regulates both bone production and breakdown. But PTH can only promote bone growth if mice have a gut full of microbes. Specifically, the microbes make a short-chain fatty acid called butyrate that facilitates this particular conversation. (Incidentally, that FGF23 made by osteocytes also acts on the parathyroid glands, tuning down their secretion of PTH.)
While scientists have uncovered many important roles for the gut microbiome in recent years, it wasn’t a given that they’d influence the skeleton, says Bonewald: “Boy, were we surprised to see effects on bone.” Now it’s clear there are plenty of complex conversations occurring between bone cells and gut microbes, and researchers are just starting to explore that complexity and what it might mean for overall health, says McCabe.
Can doctors join the conversation?
The most thrilling thing about these organ-to-organ messages, says McCabe, is that it suggests novel ways to help bone with medicines that act on different parts of the body. “We could be even more creative therapeutically,” she says.
The Centers for Disease Control and Prevention estimates that nearly 13 percent of Americans over 50 suffer from osteoporosis, and while there are several medications that slow the breakdown of bone, as well as some that speed buildup, they can have side effects and they’re not used nearly as much as they could be, says Sundeep Khosla, an endocrinologist at the Mayo Clinic in Rochester, Minnesota. That’s why he says new approaches are needed.
One obvious place to start is with the gut. Probiotics and other foods containing cultured microbes, such as the fermented milk kefir, can help to build a healthy microbiome. McCabe’s group found that a particular probiotic bacterium, Lactobacillus reuteri, protected mice from the bone loss that normally follows antibiotic treatment. Another group tried a combination of three types of Lactobacillus in post-menopausal women, the segment of the population most susceptible to osteoporosis, and those on the treatment experienced no bone loss during the yearlong study, whereas those in a placebo group did.
Hernandez has been investigating another therapeutic approach that would improve bone’s resilience, but not by adding mass or preventing breakdown. The work grew out of a series of experiments in which he used antibiotics to perturb, but not eliminate, the gut microbiome in mice. He predicted this would cause the mice to lose bone mass, but the results surprised him. “It didn’t change the density or the size of the bone,” he says, “but it changed how strong the bone was.” The bones of the antibiotic-treated animals were weak and brittle.
Investigating further, Hernandez’s team found that when mice receive antibiotics, their gut bacteria stop making as much vitamin K as they normally do, and so less of the vitamin reaches the large intestine, liver and kidneys. The result is alterations to the precise shape of mineral crystals in the bone. Hernandez is now investigating whether the source of the vitamin K — either from gut microbes or dietary sources like leafy greens — matters for bone crystallization. If people need the bacterial version, then probiotics or even fecal transplants might help, he suggests.
Karsenty’s work, meanwhile, has inspired an entirely different strategy. As he observed early on, leptin from fat cells slows bone formation via the brain. In response to leptin, the brain sends a signal that ultimately activates bone cells’ beta-adrenergic receptors, shutting off bone-building osteoblasts and stimulating bone-clearing osteoclasts.
These same beta-adrenergic receptors exist in various parts of the body, including the heart, and drugs that block them are commonly used to reduce blood pressure. To investigate whether these drugs might also prevent osteoporosis, Khosla tested a few different beta blockers in 155 post-menopausal women, and two of the drugs seemed to keep bones strong. He’s now running a larger study with 420 women; half will receive one of those drugs, atenolol, and the other half will get a placebo, for two years. The scientists will monitor them for changes in bone density in the hip and lower spine.
Khosla has another idea, based on the fact that as bone ages, it accumulates old, senescent osteocytes that produce inflammation. That inflammation, in turn, can affect the constant buildup and breakdown of bone, contributing to their imbalance in osteoporosis.
Senolytics are drugs that cause those old cells to kill themselves, and Khosla recently co-authored a summary of their potential for the Annual Review of Pharmacology and Toxicology. In a study in older mice, for example, this kind of medication boosted bone mass and strength. Khosla has another trial going, with 120 women age 70 or older, to test the ability of senolytics to increase bone growth or minimize its destruction.
Scientists still have plenty to learn about the conversations between bone and the rest of the body. With time, this research may lead to more therapies to keep not just the skeleton, but also the other conversationalists, healthy and strong.
But what’s clear already is that the skeleton is not just a nice set of mechanical supports. Bones constantly remodel themselves in response to the body’s needs, and they’re in constant communication with other parts of the body. Bone is a busy tissue with broad influence, and it’s working behind the scenes during the most basic daily activities.
So the next time you enjoy a cup of yogurt, work out or even empty your bladder, be sure to spare a moment to thank your bones for responding to microbial signals, conversing with your muscles and keeping your phosphorus supplies from going down the drain.
This article originally appeared in Knowable Magazine, an independent journalistic endeavor from Annual Reviews.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

