A tiny ‘kiss’ with mega consequences
Pop quiz: “Kissing bug” is … (A) a more amorous variant of the jitterbug, (B) another name for the mysterious cooties that middle schoolers invoke to justify their reluctance to associate with certain cute classmates, or (C) a common name for a group of insects that are accessory to the murder of 12,000 people per year.

If you answered “C”, you’d be correct (the sudden escalation in gravity may have given it away). Although “kissing bug” might conjure images of a harmless, winsome six-legged creature — perhaps a cousin of the ladybug with a heart-shaped pattern on its wings — it is actually an epithet for the far less charming Triatominae, a subfamily of the Reduviidae.
What makes these insects so odious is not their appearance (although they do exhibit a disappointing lack of heart shapes), but rather what’s inside: Triatomine bugs play host to Trypanosoma cruzi, the parasite that causes Chagas disease.
Formally described by the eponymous Brazilian physician and scientist Carlos Ribeiro Justiniano Chagas in 1908 and 1909, Chagas disease is considered a neglected tropical disease, long relegated to the periphery of scientific and medical attention since it primarily impacts impoverished populations in underdeveloped areas who don’t have much of a say in where funding for research is directed. Despite its historically low-priority status, Chagas disease casts a long shadow: An estimated 6 million to 7 million people are infected with T. cruzi worldwide, with about 12,000 dying from the disease each year, and an additional 75 million are at risk of infection.
In response to advocacy by the International Federation of Associations of People Affected by Chagas Disease, the WH designated April 14 as World Chagas Disease Day in 2019 and began observing the Day in 2020, on the 111th anniversary of the first formal Chagas disease diagnosis, made by Dr. Chagas after he examined a 2-year-old Brazilian girl named Berenice Soares de Moura.
To mark the second iteration of World Chagas Disease Day, let’s learn a bit about the disease and the strides researchers are making through work published in American Society for Biochemistry and Molecular Biology journals.
Transmission mechanisms
Like the higher-profile parasitic disease malaria, which is caused by several species of Plasmodium parasite and transmitted by infected Anopheles mosquitoes, Chagas disease has ancient origins: T. cruzi DNA has been detected in the 9,000-year-old mummified remains of a member of the Chinchorro culture, which once thrived in the coastal regions of the Atacama Desert in modern-day Peru and Chile.
Today, Chagas disease — also known as American trypanosomiasis to distinguish it from African trypanosomiasis, caused by T. brucei — is endemic in 21 Latin American countries, where it is spread primarily by kissing bugs (also known by a variety of other regional nicknames).
This moniker comes from the propensity of the nocturnal insects to bite sleeping human victims around the mouth to take a bloodmeal, but, ironically, it isn’t the “kiss” itself that transmits the parasite payload.
Unlike Plasmodium parasites and T. brucei, which have evolved to migrate to the salivary glands as they develop inside their insect hosts, T. cruzi sticks around in the gut, making its great escape in the fecal material that the insects tend to deposit around the same time as they take their meal.
“Kissing bug” is therefore something of a euphemistic misnomer, although to be fair, “dung beetle” was already taken.
In any case, this behavioral quirk works to the parasite’s great advantage: While the triatomine’s bite is not painful, it is itchy, prompting the victim to sweep the parasites — which are in their infective trypomastigote form — right into the adjacent bite wound (or a nearby mucous membrane or skin break) as they satisfy the itch.
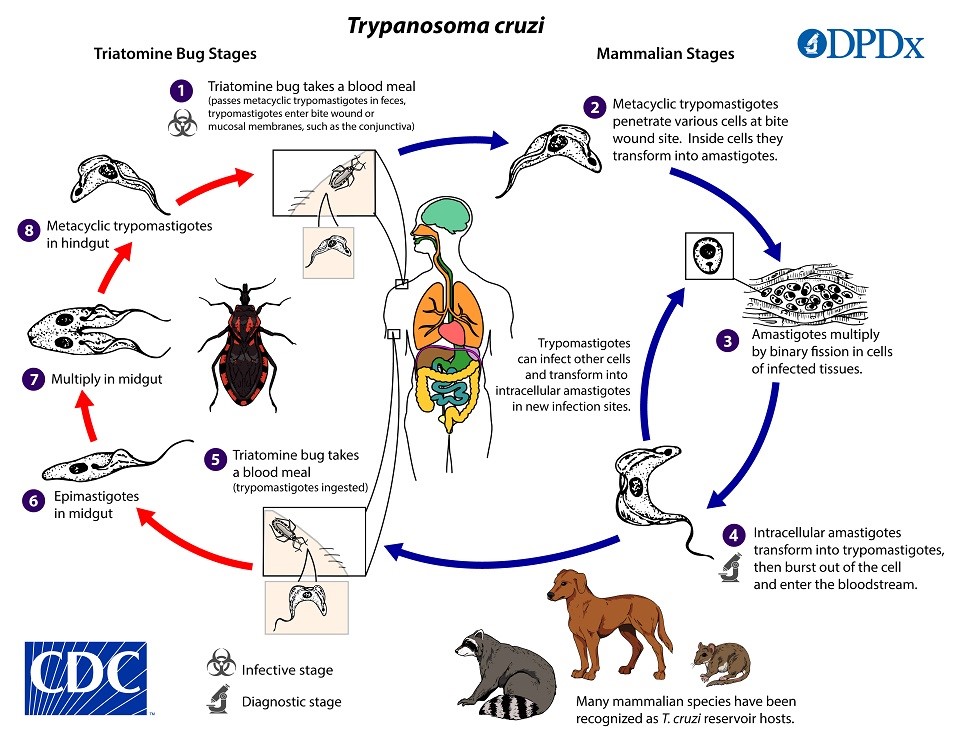
As this graphic from the CDC shows, once inoculated into the wound, the trypomastigotes enter adjacent cells and assume their intra-mammalian replicative form as amastigotes. After several rounds of multiplication by binary fission, the amastigotes change back into trypomastigotes and bust out of their host cell into the bloodstream to hitch a ride to new areas of the body, where they can infect a variety of tissue types to carry out further rounds of replication.
To ensure transmission to a new host, trypomastigotes traveling in the bloodstream can also be ingested by hungry triatomines who select an infected individual for their bloodmeal. Once inside the insect midgut, the trypomastigotes transition into another replicative state, becoming epimastigotes, and multiply before shifting back to the infective trypomastigote state in the insect’s hindgut to prepare for delivery to a new mammalian host (T. cruzi isn’t picky: the parasite can propagate inside many mammalian species besides humans).
Although T. cruzi is primarily transmitted through the kissing bug’s rather boorish habit of taking a peri-prandial bathroom break, other mechanisms of transmission that allow the parasite to skirt the requirement for an insect intermediate can occur; these include transmission via blood transfusions and organ transplants (instances of which have been reduced by improved screening of donated blood and organs) and via vertical transmission during pregnancy (the parasite can cross the placenta, although the mechanisms by which it achieves this rare feat and why it only happens around 5% of the time are not clear; this recent review article explains some of the factors that may be at play). Rarely, infection can result from consuming food or drink contaminated with parasite-laden insect excrement.
A mild acute course ...
So all these parasites running around crowding the bloodstream, hijacking cells to use as breeding grounds, and busting those cells open when they’re done have got to have some serious clinical consequences, right?
Surprisingly, the acute phase of infection — which generally lasts for around two months after the first few parasites wriggle their way into a new human host — is often asymptomatic. When symptoms do occur, they are nonspecific and can include anything from fever, headache, and lymphadenopathy to muscle pain, difficulty breathing, and chest or abdominal pain.
In some cases (< 50% of recipients of a triatomine “kiss”), characteristic nodules form on the skin around the site of infection. These nodules are known as Romana’s sign, after the Argentinian doctor who first described it, when they occur over the eyelid and as “chagomas” anywhere else.
During the acute phase of infection, the antiparasitics benznidazole and nifurtimox are highly effective at clearing the parasites. However, a lack of severe or specific symptoms and a lack of properly equipped medical facilities in endemic regions mean that the majority of patients — 90%, according to some estimates — go undiagnosed and thus untreated.
Even without treatment, the immune system usually manages to rein in the infection, dramatically reducing the level of circulating parasites.
... but deadly long-term effects
Two months of no symptoms or relatively mild ones before spontaneous resolution doesn’t sound all that bad — so where do these 12,000 deaths per year come from?
Although the immune system ultimately controls parasite replication in most patients, it turns out that this parasite has a stealth mode that prevents its complete elimination.
After the acute phase, patients enter what’s called the indeterminate form of chronic infection. Most patients — around 60% to 70% — remain in this subclinical state with no further consequences. The other 30% to 40% are not as fortunate: Years to decades after initial infection, these individuals progress to the determinate form of disease, and it is this form that takes lives.
The determinate form most commonly manifests as a dilated cardiomyopathy, or enlargement of the heart, which can lead to potentially fatal heart failure and other cardiac abnormalities, including arrythmias that can lead to sudden death.
The digestive system also takes a hit in many cases, resulting in serious complications such as enlargement of the esophagus (megaesophagus) and/or colon (megacolon). Neuropathy and stroke can also occur. The reasons for progression to clinical disease in some chronically infected patients but not others are not well understood.
Though the reasons they develop may not be clear, swollen hearts, esophagi and colons are clearly things to avoid. So what can be done to protect people from the crafty creatures that bring them about?
Because of the abundance of triatomine species that can transmit T. cruzi and the existence of a large animal reservoir, eradication is not a feasible goal. Instead, public health systems must center their efforts on prevention and early diagnosis and treatment (efficacy of the two available antiparasitics for Chagas disease seems to drop as time since infection increases).
As with most vector-mediated diseases, vector control is key to prevention. The WHO recommends insecticide treatment of homes and installation of bed nets in endemic areas to prevent triatomine bugs from bestowing their noxious “kisses.” To stem other modes of transmission, protocols are in place in many affected regions for screening blood and organ donors, treating infected women of child-bearing age before they become pregnant, and diagnosing and treating newborns with congenital Chagas disease before it becomes chronic.
Although many improvements have been made in endemic areas to stop the spread, increased migration of chronically infected individuals in recent decades means that doctors and public health officials in nonendemic regions across the globe must also be aware of these vector-independent mechanisms of Chagas disease transmission and how to prevent them.
There are also indications that the endemic region may be expanding: Once exceedingly rare, cases of vector-borne Chagas disease are becoming more common in the southern United States, a worrying trend some suspect may be a consequence of climate change.
Unveiling the secrets of T. cruzi biology
The past 112 years since Dr. Chagas announced the diagnosis of the first Chagas disease patient have brought considerable advancements in our understanding of the disease and control of its spread. However, many questions related to the pathogenesis of chronic Chagas disease and how to mitigate its debilitating consequences remain.
Finding the answers to these questions will be key to helping some of the 6 million to 7 million people chronically infected with T. cruzi, plus the thousands of newly infected individuals who join their ranks each year, avoid life-threatening illness.
It may also inform the development of a vaccine to protect the 75 million at risk of infection when vector-control measures aren’t enough.
To that end, I’ll conclude by summarizing some recent contributions to our knowledge of T. cruzi biology, all published in the Journal of Biological Chemistry, an American Society for Biochemistry and Molecular Biology peer-reviewed publication.
New insights into heme homeostasis in T. cruzi: T. cruzi cannot synthesize the molecule heme, which is essential for many biologic processes. Instead, it pilfers this critical cofactor from its mammalian or insect host via mechanisms that have not yet been fully described. More detailed knowledge of the processes involved in sensing and importing heme could inform the development of antiparasitic strategies that block access of the parasite to host-derived heme. In this article from September 2020, researchers studied the parasitic membrane protein TcHTE, which was previously demonstrated to play a role in heme transport. They showed that TcHTE protein and mRNA levels decreased in response to increasing extracellular heme concentrations, suggesting a role for TcHTE in the regulation of heme import.
Crystal structures of a potential antiparasitic drug target involved in mRNA capping: Eukaryotes cap the five-prime end of their mRNAs with a modified nucleotide to ensure stability and facilitate translation into proteins. The first step in cap formation is removal of a phosphate group from the end of a newly synthesized mRNA transcript, a process catalyzed by RNA triphosphatase. The T. cruzi RNA triphosphatase, TcCet1, differs both mechanistically and structurally from the RNA triphosphatases of either its mammalian or arthropod counterparts; this combined with the essentiality of mRNA capping for mRNA integrity and thus protein synthesis make it a good potential drug target. To understand more about how TcCet1 interacts with its substrate, researchers generated crystal structures of the enzyme complexed with an inorganic tripolyphosphate in its active site. The information they report in their article from July 2020 could inform the rational development of inhibitors to block substrate binding and prevent parasitic mRNA capping.
A control point in an RNA regulation network that modulates parasite infectivity: As detailed above, T. cruzi switches back and forth between its infective trypomastigote and replicative amastigote or epimastigote forms as it completes is complex lifecycle spanning two very different hosts. Transitioning between these states necessitates large-scale shifts in protein expression. To mediate these changes, T. cruzi relies primarily on post-transcriptional regulatory mechanisms. One such mechanism is the recruitment of a range of RNA-binding proteins (RBPs), each of which binds a specific set of functionally related mRNAs to allow them to be regulated together. In an article published in May 2019, researchers sought to explore the regulation of cell-surface glycoproteins expressed during the infective trypomastigote stages of the T. cruzi life cycle. They found that the mRNAs encoding these glycoproteins bound to an RBP called TcUBP1. Overexpression of TcUBP1 led to increased expression of these transcripts and to increased parasite infectivity, suggesting a role for TcUBP1 in mediating host cell invasion by T. cruzi trypomastigotes.
A critical enzyme for T. cruzi energy metabolism, differentiation and infectivity: In a paper published in September 2018, researchers investigated the role of the T. cruzi intramitochondrial pyruvate dehydrogenase phosphatase (TcPDP) in the parasite’s metabolism. In vertebrates, PDP is activated by mitochondrial Ca2+ uptake and acts to dephosphorylate the enzyme pyruvate dehydrogenase (PDH), which leads to PDH activation and increased ATP production. The authors showed that, like vertebrate PDPs, TcPDP is activated by Ca2+ and dephosphorylates TcPDH. Next, they created a TcPDP-knockout cell line and found that epimastigotes lacking TcPDP displayed slower growth, lower oxygen consumption rates, and impaired differentiation into infective trypomastigotes compared to control cells with functional TcPDP. Similarly, trypomastigotes without TcPDP were unable to infect host cells efficiently. These results highlighted the critical role of TcPDP in T. cruzi physiology.
The switch from the replicative epimastigote to infective trypomastigote state in the triatomine gut requires a period of arrested cell division. In their article published in May 2017, another set of researchers used targeted metabolomics to compare the levels of metabolic intermediates involved in energy metabolism in actively growing vs. growth-arrested epimastigotes. They found that the parasites adapted to nutrient-poor conditions by switching their main fuel source from energy-rich metabolites such as glucose to more energy-poor amino acids. Their results suggested that T. cruzi possesses a high degree of metabolic plasticity that it likely relies upon to adapt to the many environments it encounters during its life cycle; identification of critical control points that enable this plasticity could inform development of new antiparasitic agents.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

