From the journals: March 2019
We offer a selection of recent papers on a variety of topics from the Journal of Biological Chemistry, the Journal of Lipid Research and Molecular & Cellular Proteomics.
How to tackle stress
The tumor suppressor protein p73 is known to induce apoptosis in response to stress, but the details of this pathway are poorly understood. In a paper in the Journal of Biological Chemistry, Mi-Kyung Yoon and colleagues from the Korea Research Institute of Bioscience and Biotechnology and the Korea Research Institute of Chemical Technology show that p73-mediated apoptosis occurs via a mitochondrial pathway. Moreover, the process requires an interaction between a p73 domains and a noncanonical site on the anti-apoptotic protein Bcl-XL. This study unexpectedly expands the known modes of Bcl-XL recognition and reports a new apoptotic pathway that may be relevant for cancer treatment.
Dysregulated signaling in ovarian cancer
High-grade serous carcinoma, or HGSOC, is the most aggressive and common form of ovarian cancer, accounting for about 70 percent of all cases. Studies have shown that post-translational modifications, or PTMs, play a role in reprogramming signaling networks that contribute to the cancerous phenotype. However, PTMs are more challenging to identify than genetic or proteomic changes.
A study in Molecular & Cellular Proteomics uses a new human proteome microarray-based approach to identify dysregulated PTMs associated with ovarian tumors, providing insight into PTM alterations in tumor samples. Guang Song and colleagues at Johns Hopkins University established tumor lysate-based reactions to identify multiple PTMs in cancer samples. They performed kinase phosphorylation reactions with total tissue lysates on an array of human proteins and then identified phosphorylated substrates. They looked for activated kinases upstream of these substrates to identify the enzymes capable of producing the PTM and thus possibly involved in cell signaling dysregulation. They specifically focused on tyrosine phosphorylation for their analysis and identified 19 tyrosine kinases that may play a role in signaling pathway dysregulation observed in HGSOC.
Their method provides a novel means to identify PTMs in tumor samples, providing insight into dysregulated signaling networks that may contribute to cancer.
Understanding the lipid situation in bone cancer
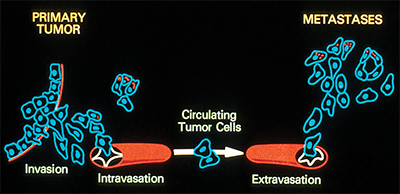 This illustration shows the process by which cancer metastasizes.Wikimedia Commons Osteosarcoma is the most common form of primary bone cancer in humans. Most people diagnosed with the disease are under the age of 25. Treatment is aggressive, consisting of tumor excision and multiple forms of chemotherapy. Even then, the average five-year survival rate is 65 percent at the non-metastatic stage and 20 percent after metastasis. Development of new therapeutic approaches requires a better understanding of the metabolic pathways involved in the development and progression of osteosarcoma.
This illustration shows the process by which cancer metastasizes.Wikimedia Commons Osteosarcoma is the most common form of primary bone cancer in humans. Most people diagnosed with the disease are under the age of 25. Treatment is aggressive, consisting of tumor excision and multiple forms of chemotherapy. Even then, the average five-year survival rate is 65 percent at the non-metastatic stage and 20 percent after metastasis. Development of new therapeutic approaches requires a better understanding of the metabolic pathways involved in the development and progression of osteosarcoma.
Significant research has been done to explore the transcriptomics and proteomics of various cancer types, but the lipidomic profile of osteosarcoma is poorly understood. Lipids are an important component of the cell machinery both structurally and functionally. In fact, dysregulation of lipid metabolism has been associated with breast and prostate cancer.
Aditi Das and her group at the University of Illinois Urbana-Champaign have compared the lipid profiles of metastatic and non-metastatic osteosarcoma cells to that of normal fetal osteoblast cells to better understand the mechanism of bone tumor formation and metastasis. Their findings were published in the Journal of Lipid Research.
Using high-throughput assays, the group has identified 15 distinct classes of lipids that are differentially expressed in cancerous cells compared to normal bone cells; these include phospholipids, glycolipids and cholesterol. Diacylglycerol was overexpressed in metastatic osteoblasts such that blocking diacylglycerol synthesis reduced cell viability and migration in metastatic osteosarcoma.
These findings help the researchers understand how the lipid profile is altered when cells are metabolically reprogrammed to support uncontrolled growth and proliferation and, in the future, might pave the way for alternative therapeutic approaches for treating pre-metastatic osteosarcoma.
— Isha Dey
Cholesterol biosynthesis in the liver
Long noncoding RNAs, or lncRNAs, are at least 200 nucleotide-long RNA sequences that do not translate into proteins. However, they have been associated with regulation of gene expression and developmental processes as well as with various disease states.
A study by Wei Li and his group at Wenzhou Medical University in China published in the Journal of Lipid Research has identified one such lncRNA as an important player in cholesterol biosynthesis. Hepatic transcriptomic analysis revealed more than three dozen lncRNAs were differently expressed in mice fed with a high-fat diet (a model system for cholesterol synthesis and fatty liver disease) compared with mice fed a standard chow diet. A correlation analysis of lncRNA and protein-coding RNA interpreted the functions of the identified lncRNAs. Connecting the lncRNAs to metabolic pathways using cell-based assays, the authors identified an lncRNA called NONMMUG027912 that was regulated by PPAR-alpha, a key regulator of lipid metabolism in the liver, such that reducing NONMMUG027912 levels in liver cells increased cholesterol biosynthesis.
This study provides a better understanding of the mechanism of action of lncRNAs in lipid metabolism and liver diseases and identifies a new candidate for maintaining cholesterol homeostasis.
An insect toxin grabs with two hands
When farmers want to avoid using pesticides, they often turn to transgenic crops containing the crystalline Cry toxins produced by Bacillus thuringiensis, or Bt. These plants are used worldwide because the concentrations of toxins in plants only harm the insects that are feeding directly on them. In 2017, Bt crops were cultivated on more than 240 million acres. However, pests including the pink bollworm and Indian-meal moth have evolved rapidly to resist the toxins.
To improve the efficacy of the Cry toxins, which kill by binding to insect midgut receptors, Arlen Peña-Cardeña and colleagues at the Universidad Nacional Autónoma de México investigated the binding activity of Cry1Ab’s C-terminal region. This region previously was believed to be an inactive part of the Cry1Ab toxin that just needed to be cleaved by proteases before the toxin could begin binding.
In a study published in the Journal of Biological Chemistry, the researchers found that Cry1ab’s C-terminal region provides additional binding sites for alkaline phosphatase and aminopeptidase insect receptors but not for cadherin, a target of the main toxin. By discovering that the components of Cry1ab target insect guts with a variety of receptors, the researchers may be able to alter the specificity and increase the toxicity of the proteins.
Scorpion venoms vanquish viruses
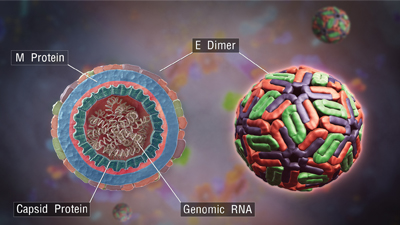 Dengue viruses are members of the family Flaviviridae, which also includes Zika virus, West Nile virus, yellow fever virus and hepatitis C virus.Girish Khera/Scientific AnimationsThe four dengue viruses, which cause fevers on their own and hemorrhages in concert, emerged over eight centuries in Southeast Asia and Africa, where they became endemic with the aid of mosquitoes and occasional nonhuman mammalian reservoirs. As shipping containers and trade agreements seemed to shrink the globe after World War II, they also opened up dozens of other tropical and subtropical countries to the dengue-carrying mosquitoes Aedes aegypti and Aedes albopictus, which arrived hidden away in cargo ships.
Dengue viruses are members of the family Flaviviridae, which also includes Zika virus, West Nile virus, yellow fever virus and hepatitis C virus.Girish Khera/Scientific AnimationsThe four dengue viruses, which cause fevers on their own and hemorrhages in concert, emerged over eight centuries in Southeast Asia and Africa, where they became endemic with the aid of mosquitoes and occasional nonhuman mammalian reservoirs. As shipping containers and trade agreements seemed to shrink the globe after World War II, they also opened up dozens of other tropical and subtropical countries to the dengue-carrying mosquitoes Aedes aegypti and Aedes albopictus, which arrived hidden away in cargo ships.
Today, nearly 2.5 billion people live in areas where dengue transmission is a risk. Despite preclinical successes, the leading antiviral and vaccine candidates for treating and preventing dengue, balapiravir and dengvaxia, were both found to have dangerous side effects.
Zhijian Cao and his colleagues at Wuhan University in China may have a new antiviral candidate. They recently found that venom peptides from the scorpion Euscorpiops validus, native to southern China, are effective at blocking dengue virus type 2 from entering bacterial cells. They published their results in the Journal of Biological Chemistry.
The researchers tested a purified version of the venom peptide Ev32, called rEv32, against dengue virus type 2 in both Escherichia coli and Staphylococcus aureus. There, it was able to raise the pH of acidic organelles within the bacteria, preventing the viruses’ pH-dependent membranes from fusing with the inner bacterial membranes. This effectively trapped the viruses in cells after they already had entered and replicated, shutting down the viral infections.
Subsequent experiments found that a purified form of the venom, rEV32, acted against hepatitis C virus, Zika virus and herpes simplex virus 1, which all have cellular entry processes similar to dengue. The researchers hope to begin testing rEv32 as an antiviral drug candidate in the near future.
— John Arnst
The secretome of a Gram-positive bacteria
Protein secretion is an essential biological process. Studying the array of proteins secreted by a bacterial or eukaryotic cell, called the secretome, can give insight into its response to surrounding stimuli. A paper published in Molecular & Cellular Proteomics describes the secretome dynamics of the Gram-positive bacterium Streptomyces lividans. A team led by Tassos Economou at the Rega Institute in the Katholieke Universiteit Leuven, Belgium, used mass spectrometry and transcriptomics to analyze protein secretion across a variety of growth conditions. They identified several so-called housekeeping proteins that were secreted in stable amounts irrespective of growth conditions or genetic background, suggesting they are essential for cell proteostasis. They also observed that bacteria growing more slowly, which therefore had lower cell mass, secreted higher amounts of protein. They hypothesize that this is due to shuttling of metabolic intermediates toward secretion instead of cellular growth, thereby linking metabolism and secretion. Additionally, not all changes in the quantity of individual secreted proteins were explained by changes in transcription. This suggests another level of downstream regulation that has not been identified. Their findings have implications for how secretome, proteome and metabolome studies can be integrated to better understand regulation of vital cell processes.
A dead domain creates dimers
Mutations in a group of magnesium transporters called the cyclin N family are associated with several diseases, including familial primary hypomagnesemia, a blood disorder, and Jalili syndrome, which affects the eyes. A better understanding of how these transporters function could potentially aid in treating these diseases. Yu Seby Chen and colleagues at McGill University in Canada and Osaka University in Japan now have characterized one particularly mysterious part of the proteins: the cyclic nucleotide binding homology domain. The authors, publishing in the Journal of Biological Chemistry, found that the domain is required for transporter function but that mutations may be responsible for it losing the ability to bind nucleotides.
Instead, these domains cause protein dimerization in vitro, with dimer strength inversely correlating with transporter activity. The authors postulate that these domains therefore may inhibit protein function, providing a new framework to evaluate disease-linked sequences.
Phosphoproteomics correlate cellular effects of chemotherapy
 HeLa cells expressing Histone H2B-GFP fixed and stained for F-actin with rhodamine-phalloidin.UraniumMonkey/Wikimedia CommonsSynthetic sphingolipids can be used as chemotherapy agents to starve cancer cells. These lipid therapeutics trigger downregulation of nutrient transporters and block lysosomal fusion events to kill the cell. It is known that sphingolipids activate protein phosphatase 2A, or PP2A, and negatively regulate signaling pathways that promote expression of nutrient transporters.
HeLa cells expressing Histone H2B-GFP fixed and stained for F-actin with rhodamine-phalloidin.UraniumMonkey/Wikimedia CommonsSynthetic sphingolipids can be used as chemotherapy agents to starve cancer cells. These lipid therapeutics trigger downregulation of nutrient transporters and block lysosomal fusion events to kill the cell. It is known that sphingolipids activate protein phosphatase 2A, or PP2A, and negatively regulate signaling pathways that promote expression of nutrient transporters.
Pierre Thibault and colleagues from the Université de Montréal in Canada used quantitative phosphoproteomics to determine how PP2A alters cell fusion events in a mouse cell line. They treated the cells with a synthetic sphingolipid called SH-BC-893 that has been investigated pre-clinically as a means to target and kill cancer cells. They also separately treated the cells with a related lipid, ceramide, one that is known to stimulate PP2A but isn’t used as a chemotherapy agent, and also with a specific PP2A inhibitor. Using metabolic labeling, they identified phosphorylation sites that were regulated by the treatments and therefore could be putative PP2A substrates.
The researchers’ analysis confirmed that SH-BC-893 does affect PP2A activity, and they identified several putative PP2A substrates. A significant proportion of these targets were found to be involved in actin cytoskeleton organization and cell migration pathways.
Analyses of these substrates suggest that they may contribute to the intracellular trafficking defects observed in cells treated with sphingolipids. They further identified that treatment with ceramide, but not SH-BC-893, dysregulates two proteins called Akt and Gsk3b, explaining why only SH-BC-893 produces a lysosomal fusion defect. Their work, recently published in Molecular & Cellular Proteomics, demonstrates the utility of using dynamic phosphoproteomics to correlate signaling events with cellular phenotype.— Courtney Chandler
Neuronal GIRK currents and blood cholesterol level
G protein-mediated inwardly rectifying potassium channels, or GIRKs, translate chemical transmissions in the brain into electrical signals at the post-synaptic regions of hippocampal neurons to mediate the effects of inhibitory neurotransmitters. Another component essential for normal neuronal activity is cholesterol, which forms up to 50 percent of the membrane lipids. About 20 percent of the total cholesterol in the human body is in the brain. Abnormal cholesterol levels in the brain have been associated with such neurodegenerative conditions as Alzheimer’s disease.
A recent collaborative study by the University of Illinois at Chicago, the University of Tennessee Health Science Center and the National Institutes of Health established the role of statin therapy (used to treat high cholesterol levels in the blood) on hippocampal GIRK currents. The findings were published in the Journal of Lipid Research. Led by Avia Rosenhouse-Dantsker and Anna N. Bukiya, the group used a rat model to show that a high-cholesterol diet increased hippocampal cholesterol levels and neuronal GIRK currents. However, a high-cholesterol diet combined with statin therapy counteracted the effect. This finding is interesting, as researchers expected the cholesterol pool in the brain to be shielded from blood cholesterol fluctuations.
The study sheds new light on the regulation of ion channel function by lipids. It also provides a better understanding of the multifaceted effects of statin therapy in the brain.
This article was corrected March 12. In the original version, Anna Bukiya’s affiliation was incorrect. She is an associate professor at the University of Tennessee Health Science Center.
A selection simulation
Newborn babies often are tested for a condition called phenylketo- nuria, in which deleterious mutations to phenylalanine hydroxylase, or PAH, lead to elevated levels of phenylalanine, which can result in intellectual disabilities, seizures and other problems. Phenylalanine acts as an allosteric regulator of PAH, but the mechanism is not fully understood. Yunhui Ge and colleagues from Temple and Drexel Universities used computational modeling approaches to simulate interactions between Phe and its binding site in PAH. The simulations implicated a conformational selection mechanism in which a gate in the protein must open to allow Phe binding. These mechanistic details, published in the Journal of Biological Chemistry, could offer new directions for therapeutic development.
Correction
The item title “Neuronal GIRK currents and blood cholesterol level” should have stated that Anna Bukiya of the University of Tennessee Health Science Center and Avia Rosenhouse-Dantsker of the University of Illinois at Chicago led a team that published in the Journal of Lipid Research.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.