From the journals: April 2019
We offer a selection of recent papers on a variety of topics from the Journal of Biological Chemistry, the Journal of Lipid Research and Molecular & Cellular Proteomics.
Blood and aggression in crayfish
Transglutaminase is a cross-linking enzyme involved in diverse cellular functions including blood coagulation and communication between cells and the extracellular matrix. Different isoforms of the enzyme have different substrate specificities and are expressed differentially across tissues and cell types. Kingkamon Junkunlo and colleagues at Uppsala University examined the role of transglutaminase in hematopoiesis in crayfish. They found that inhibiting transglutaminase not only increased the number of circulating blood cells but also unexpectedly made crayfish move less and display less aggressive behavior. This link between hematopoiesis and the nervous system controlling behavior may provide insights into diseases involving defects in motor functions. The study was published in the Journal of Biological Chemistry.
Early markers of Alzheimer’s disease
Alzheimer’s disease is characterized by neurodegeneration, including synapse loss, and preclinical markers of Alzheimer’s and other neuronal disorders have been elusive. These markers someday could be used to diagnose patients before symptoms start.
Alberto Lleo, Olivia Belbin and colleagues at the Hospital de la Santa Creu i Sant Pau in Barcelona, Spain, used a mass spectrometry-based proteomic technique to determine which proteins in cerebrospinal fluid, or CSF, samples change prior to the onset of Alzheimer’s symptoms. Their results were published in the journal Molecular & Cellular Proteomics.
The researchers first compared CSF proteins in Alzheimer’s patients to those in people without the disease. Next, they narrowed the set down to CSF proteins from synapses, identified by comparing the data set to synaptic proteins established in the literature. Nine were chosen for monitoring changes in expression levels in Alzheimer’s patients.
Using a proteomics technique called selected reaction monitoring, the researchers found that while some marker proteins were high throughout the onset of disease, several proteins were lower in CSF from presymptomatic patients than in healthy individuals but then were elevated where neurodegeneration occurred. Lleo and colleagues described this as a “biphasic profile” and concluded that those decreased proteins could be early biomarkers of synapse loss that could be used to identify individuals with Alzheimer’s disease before irreversible neurodegeneration becomes widespread.
Distinguishing near-identical enzymes to help treat Cushing’s disease
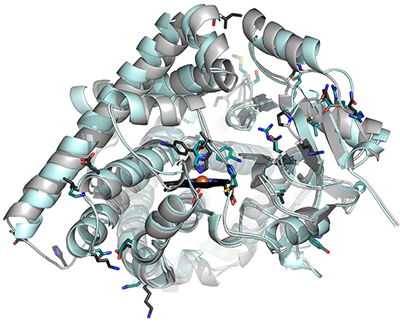 A structural comparison of CYP11B1 (cyan) and CYP11B2 (gray). Darker sticks represent nonidentical amino acids.brixius-anderko et al.The stress hormone cortisol is produced in the adrenal glands but has important duties throughout the body, from nutrient metabolism to immune suppression. Excessive production of this hormone can have devastating consequences, however, such as Cushing’s disease, with symptoms that include weakened immune responses, rapid weight gain and osteoporosis.
A structural comparison of CYP11B1 (cyan) and CYP11B2 (gray). Darker sticks represent nonidentical amino acids.brixius-anderko et al.The stress hormone cortisol is produced in the adrenal glands but has important duties throughout the body, from nutrient metabolism to immune suppression. Excessive production of this hormone can have devastating consequences, however, such as Cushing’s disease, with symptoms that include weakened immune responses, rapid weight gain and osteoporosis.
In a study in the Journal of Biological Chemistry, Simone Brixius–Anderko and Emily Scott at the University of Michigan shed light on unique structural features of the cortisol-producing enzyme cytochrome P450 11B1, or CYP11B1, that could aid researchers in designing selective drugs for Cushing’s disease treatment.
For years, researchers have thought of CYP11B1 as a prime drug target for Cushing’s; however, 93 percent of the enzyme’s amino acid sequence is shared with CYP11B2, which is essential for the production of aldosterone, a hormone central to the regulation of blood pressure. Structural differences between these close relatives have not been determined, so researchers have difficulty developing drugs that will specifically inhibit CYP11B1 and reduce cortisol levels without also disrupting CYP11B2’s aldosterone-producing activities.
Using an X-ray-based technique, the researchers found the crystal structure of each enzyme bound to fadrozole, a breast cancer drug that inhibits the estrogen-producing CYP19A1. They identified considerable differences between the orientation and placement of certain residues within the enzymes. Because of these distinctions, the enzymes favored mirrored shapes, or enantiomers, of the drug. CYP11B1’s structure accommodated the shape of the left-handed, or S, enantiomer of fadrozole, while CYP11B2 was a better fit for the right-handed version.
By identifying CYP11B1’s strong preference for the S enantiomer, this study could aid in the development of more selective inhibitors that would treat Cushing’s disease without causing unwanted effects, such as reduced aldosterone, in patients.
— Jonathan Griffin
The new old cause of gallstones
A person living in the U.S. has a 10 to 20 percent chance of developing stones in their gallbladder. Yet up to 80 percent of affected individuals will have no symptoms. The remaining 20 percent may experience biliary pain or other complications requiring costly interventions.
In a paper in the Journal of Lipid Research, Mats Rudling and colleagues at the Karolinska Institute, Sweden, describe new insight into the cause of gallstones.
The stones develop when gallbladder bile is supersaturated with cholesterol, one of three major lipid components in bile, with bile acids and phospholipids being the other two. Traditionally, it is thought that excessive hepatic secretion of cholesterol is the main cause for such supersaturation. Rudling’s team used unpublished data from a previous study to show that a shortage of bile acids is probably the major and most common reason why gallbladder bile is supersaturated with cholesterol in gallstone patients. Furthermore, the researchers used 13 previously published studies from 11 countries to corroborate their findings. Future studies are needed to investigate the cause(s) of reduced bile acid levels.
Discovering a new pool of iron
Like many elements, iron is a double-edged sword. It is an essential cofactor but can pose a danger to cells by catalyzing the formation of reactive oxygen species. In a paper in the Journal of Biological Chemistry, Joshua Wofford and colleagues at Texas A&M University describe how they revised previous estimates of iron usage in cells by using multiple methods to inventory total iron content and characterize the distribution of different iron species in Escherichia coli. They discovered a previously unknown low-molecular-mass Fe(II) pool in the cytosol and suggested that this pool might be prevented from reacting with oxygen by a shield of membrane-bound iron-rich respiratory complexes.
Is an over-the-counter sleep aid the next diet fad?
 The white fat running through red muscle, also known as intramuscular fat, or IMF, is made of lipids deposited in fat cells, called adipocytes, which affect the flavor of animal meat as well as insulin sensitivity in humans.michael c. berch/wikimedia commons Steak connoisseurs are familiar with the marble pattern of white fat running through raw red flesh. The white fat inside the muscle, also known as intramuscular fat, or IMF, is inside adipose cells, called adipocytes, that are located between the skeletal muscle fibers. In research published in the Journal of Lipid Research, Kaiqaing Liu and colleagues at Nanjing Agricultural University, China, investigated how melatonin, a circadian rhythm-regulating hormone involved in sleep, affects how IMF is deposited and showed that long-term melatonin treatment reduces IMF deposition. These results are relevant for farm animal breeding where fat content affects flavor and for human “globesity” — the global epidemic of overweight and obesity.
The white fat running through red muscle, also known as intramuscular fat, or IMF, is made of lipids deposited in fat cells, called adipocytes, which affect the flavor of animal meat as well as insulin sensitivity in humans.michael c. berch/wikimedia commons Steak connoisseurs are familiar with the marble pattern of white fat running through raw red flesh. The white fat inside the muscle, also known as intramuscular fat, or IMF, is inside adipose cells, called adipocytes, that are located between the skeletal muscle fibers. In research published in the Journal of Lipid Research, Kaiqaing Liu and colleagues at Nanjing Agricultural University, China, investigated how melatonin, a circadian rhythm-regulating hormone involved in sleep, affects how IMF is deposited and showed that long-term melatonin treatment reduces IMF deposition. These results are relevant for farm animal breeding where fat content affects flavor and for human “globesity” — the global epidemic of overweight and obesity.
In humans, IMF content affects insulin resistance and type 2 diabetes, with the reduction of IMF content improving insulin sensitivity of muscle tissue. Several previous studies using cells and laboratory animals also have shown that melatonin affects fat deposition, but the observed effects differed by study. Liu and colleagues found consistent results in pig fat cells and mice, and they proposed a mechanism for melatonin’s effect on IMF deposition. Specifically, the researchers showed that melatonin treatment inhibited pig intramuscular preadipocytes’ proliferation by arresting the cell cycle in a way that varied according to dose and time.
Furthermore, the size of deposited lipid droplets in melatonin-treated preadipocytes was smaller while the expression of lipolytic enzymes was increased. Additional experiments showed that melatonin likely acts via an increase in uptake through upregulation of its receptor, MT2, and involves ERK1/2 and protein kinase A signaling pathway. Consistent with these observations in cultured cells, mice injected with melatonin had lower body weight and lower fat deposition than control mice.
This research suggests that doctors and personal trainers may soon recommend melatonin supplements in addition to exercise and caloric restrictions to prevent and treat obesity and related diseases.
— Nathalie Gerassimov
DNA repair pathway resolved
DNA repair is a critical function, and cells have multiple ways of carrying it out. One efficient method of DNA repair is transcription-coupled repair, which is carried out as DNA is being transcribed. Previous research provided conflicting evidence as to whether transcription-coupled repair worked on DNA sequences encoding ribosomal RNA; this was unclear because rRNA and protein-coding genes are transcribed by different RNA polymerases in eukaryotes. Yanyan Yang and colleagues at the University of North Carolina used excision repair sequencing with single-nucleotide resolution to investigate this question. They found that transcribed and non-transcribed sections of ribosomal DNA are repaired at the same rates, suggesting that ribosomal DNA is repaired by global repair mechanisms, not transcription-coupled repair. The study was published in the Journal of Biological Chemistry.
Glycoproteins and food poisoning
The gastrointestinal bacterium Campylobacter jejuni, a major cause of food poisoning, causes abdominal cramps and inflammation and is transmitted through contaminated water or poultry. C. jejuni uses a sophisticated system to attach oligosaccharide chains to the nitrogen in some amino acids. The system involves multiple steps carried out by the pgl cluster, which codes for proteins that act as a glycoprotein assembly line. Researchers at the University of Sydney generated C. jejuni bacteria without a key gene in this cluster and performed proteomic analysis to determine the importance of N-glycosylation in pathogenesis. Their work was published in the journal Molecular & Cellular Proteomics.
Researchers found that without the pglB protein, C. jejuni no longer could recover from extreme cold or heat as normal bacteria could. In addition, C. jejuni switched which amino acids it used most, decreased levels of certain transport proteins and could no longer migrate toward some metabolic substrates.
Without glycosylation, certain proteins within the glycoproteome were downregulated, and an enzyme involved in utilization of nitrogen was less active. This was specific; a strain without the pgl cluster had restored activity of this enzyme when researchers added the pgl cluster back. Because protein glycosylation in C. jejuni was found to affect many pathways, it could be a way to stop this gastrointestinal bacterium.
How an upstream enhancer regulates a protein’s expression
GPIHBP1 is a protein that shuttles lipoprotein lipase, or LPL, from capillary endothelial cells to capillary lumen, where it can process triglyceride-rich lipoproteins. Lack of GPIHBP1 prevents LPL relocation to the capillary lumen, impairing plasma triglyceride hydrolysis and leading to severe hypertriglyceridemia (high blood levels of triglycerides — a risk factor for atherosclerosis).
In a paper published in the Journal of Lipid Research, Christopher Allan and colleagues at the universities of California, Michigan and Arizona describe an upstream enhancer that regulates GPIHBP1 expression in a tissue-specific manner. Deletion of the relevant DNA segment using CRISPR/Cas9 genome editing reduced GPIHBP1 expression by more than 90 percent in the liver and by about 50 percent in the heart and brown adipose tissue, but that was wasn’t enough to block LPL relocalization to the capillary lumen and cause hypertriglyceridemia. Future studies may investigate why the enhancer deletion had such strikingly different effects in the liver versus the heart and focus on other unknown players in the regulation of GPIHBP1 expression. It is likely that other GPIHBP1 enhancers were present, which contributed to the tissue-specific changes in expression.
How a gut pathogen sidesteps antibiotic treatment
 Klebsiella pneumoniae, shown with an immune cell, evades antibiotic treatment by altering its proteome.wikimedia commonsHospital-acquired antibiotic-resistant infections occur in hospitals and clinics worldwide. Klebsiella pneumoniae is a Gram-negative bacterium that invades the gastrointestinal and respiratory tracts, causing a range of diseases. The ability to cling to medical devices makes it especially harmful in those hospital settings where antibiotic resistance is common.
Klebsiella pneumoniae, shown with an immune cell, evades antibiotic treatment by altering its proteome.wikimedia commonsHospital-acquired antibiotic-resistant infections occur in hospitals and clinics worldwide. Klebsiella pneumoniae is a Gram-negative bacterium that invades the gastrointestinal and respiratory tracts, causing a range of diseases. The ability to cling to medical devices makes it especially harmful in those hospital settings where antibiotic resistance is common.
Sarah L. Keasey and colleagues at the U.S. Army Medical Research Institute of Infectious Diseases examined the gastrointestinal pathogen K. pneumoniae from a lethal outbreak in laboratory monkeys to determine which pathways were altered when standard antibiotics were used. They published their work in the journal Molecular & Cellular Proteomics.
When the researchers used a panel of antibiotics on bacterial cultures isolated from the infected monkeys, they found differing growth patterns depending on the treatment used. They then did a proteomics study using streptomycin and doxycycline alone and in combination. These two antibiotics both target the ribosome but have very different chemical structures, and researchers found differences in bacterial enzymes involved in biosynthesis. Next, since antibiotics must first cross the bacterial cell wall, the researchers examined changes in transport protein levels, some of which decreased with treatment, indicating that bacteria could change their proteome to prevent antibiotic entry.
As transport protein changes also meant certain sugars couldn’t enter cells, researchers found that the bacteria altered their fuel sources to compensate. In addition, the capsule that surrounds K. pneumoniae increased its composition in cells treated with doxycycline but not those treated with streptomycin, indicating they have different mechanisms of resistance. When these antibiotics were combined, researchers found that treated cells had lowered transport of certain molecules and increased levels of membrane proteins. After repeated doses, as in a clinic, K. pneumoniae recovered and its growth rate increased over time, signaling resistance to the combination.
Knowing which pathways bacteria use to evade antibiotics is therefore key to developing effective treatments for these pathogens.
— Dawn Hayward
Finding ligands using phage
USP11 is a human deubiquitinase that regulates DNA double-strand break repair and cellular stress responses; it is dysregulated in pancreatic and ovarian cancers. Unlike other ubiquitin-specific proteases, USP11’s peptide-binding sites were unknown. Anastasios Spiliotopoulos and colleagues at the University of Nottingham combined phage display library screening with next-generation sequencing to discover USP11-interacting peptide motifs. They found a binding site that modulates USP11’s DNA repair function and developed a ligand that could be used to arrest the cell cycle. This strategy, published in the Journal of Biological Chemistry, could be used to develop new biochemical tools.
Selectivity hinges on a hinge site
Despite being structurally similar, caspases are highly selective for specific substrates. In a study published in the Journal of Biological Chemistry, Derek McPherson and colleagues at the University of Massachusetts searched for secondary binding sites in caspases that could help explain this selectivity. They discovered an exosite in caspase-6 composed of a tri-arginine patch in the hinge between the core caspase domain and a disordered N-terminal domain. Since caspase-6 plays a unique role among apoptotic caspases in neurodegeneration, modulating this substrate-binding site could be therapeutically valuable for conditions such as Alzheimer’s and Huntington’s disease.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Building better tools to decipher the lipidome
Chemical engineer–turned–biophysicist Matthew Mitsche uses curiosity, coding and creativity to tackle lipid biology, uncovering PNPLA3’s role in fatty liver disease and advancing mass spectrometry tools for studying complex lipid systems.




.jpg?lang=en-US&width=300&height=300&ext=.jpg)