Vampire viruses prey on other viruses to replicate themselves
Have you ever wondered whether the virus that gave you a nasty cold can catch one itself? It may comfort you to know that, yes, viruses can actually get sick. Even better, as karmic justice would have it, the culprits turn out to be other viruses.
Viruses can get sick in the sense that their normal function is impaired. When a virus enters a cell, it can either go dormant or start replicating right away. When replicating, the virus essentially commandeers the molecular factory of the cell to make lots of copies of itself, then breaks out of the cell to set the new copies free.
Sometimes a virus enters a cell only to find that its new temporary dwelling is already home to another dormant virus. Surprise, surprise. What follows is a battle for control of the cell that can be won by either party.
But sometimes a virus will enter a cell to find a particularly nasty shock: a viral tenant waiting specifically to prey on the incoming virus.
I am a bioinformatician, and my laboratory studies the evolution of viruses. We frequently run into “viruses of viruses,” but we recently discovered something new: a virus that latches onto the neck of another virus.
A world of satellites
Biologists have known of the existence of viruses that prey on other viruses — referred to as viral “satellites” — for decades. In 1973, researchers studying bacteriophage P2, a virus that infects the gut bacterium Escherichia coli, found that this infection sometimes led to two different types of viruses emerging from the cell: phage P2 and phage P4.
Bacteriophage P4 is a temperate virus, meaning it can integrate into the chromosome of its host cell and lie dormant. When P2 infects a cell already harboring P4, the latent P4 quickly wakes up and uses the genetic instructions of P2 to make hundreds of its own small viral particles. The unsuspecting P2 is lucky to replicate a few times, if at all. In this case, biologists refer to P2 as a “helper” virus, because the satellite P4 needs P2’s genetic material to replicate and spread.
Subsequent research has shown that most bacterial species have a diverse set of satellite-helper systems, like that of P4-P2. But viral satellites are not limited to bacteria. Shortly after the largest known virus, mimivirus, was discovered in 2003, scientists also found its satellite, which they named Sputnik. Plant viral satellites that lurk in plant cells waiting for other viruses are also widespread and can have important effects on crops.
Viral arms race
Although researchers have found satellite-helper viral systems in pretty much every domain of life, their importance to biology remains underappreciated. Most obviously, viral satellites have a direct impact on their “helper” viruses, typically maiming them but sometimes making them more efficient killers. Yet that is probably the least of their contributions to biology.
Satellites and their helpers are also engaged in an endless evolutionary arms race. Satellites evolve new ways to exploit helpers and helpers evolve countermeasures to block them. Because both sides are viruses, the results of this internecine war necessarily include something of interest to people: antivirals.
Recent work indicates that many antiviral systems thought to have evolved in bacteria, like the CRISPR–Cas9 molecular scissors used in gene editing, may have originated in phages and their satellites. Somewhat ironically, with their high turnover and mutation rates, helper viruses and their satellites turn out to be evolutionary hot spots for antiviral weaponry. Trying to outsmart each other, satellite and helper viruses have come up with an unparalleled array of antiviral systems for researchers to exploit.
MindFlayer and MiniFlayer
Viral satellites have the potential to transform how researchers understand antiviral strategies, but there is still a lot to learn about them. In our recent work, my collaborators and I describe a satellite bacteriophage completely unlike previously known satellites, one that has evolved a unique, spooky lifestyle.
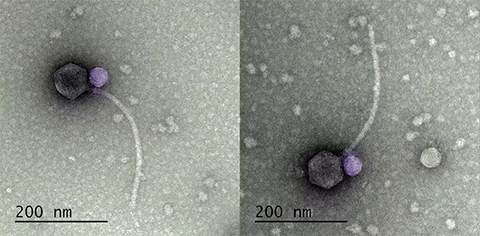
Undergraduate phage hunters at the University of Maryland, Baltimore County, isolated a satellite phage called MiniFlayer from the soil bacterium Streptomyces scabiei. MiniFlayer was found in close association with a helper virus called bacteriophage MindFlayer that infects the Streptomyces bacterium. But further research revealed that MiniFlayer was no ordinary satellite.
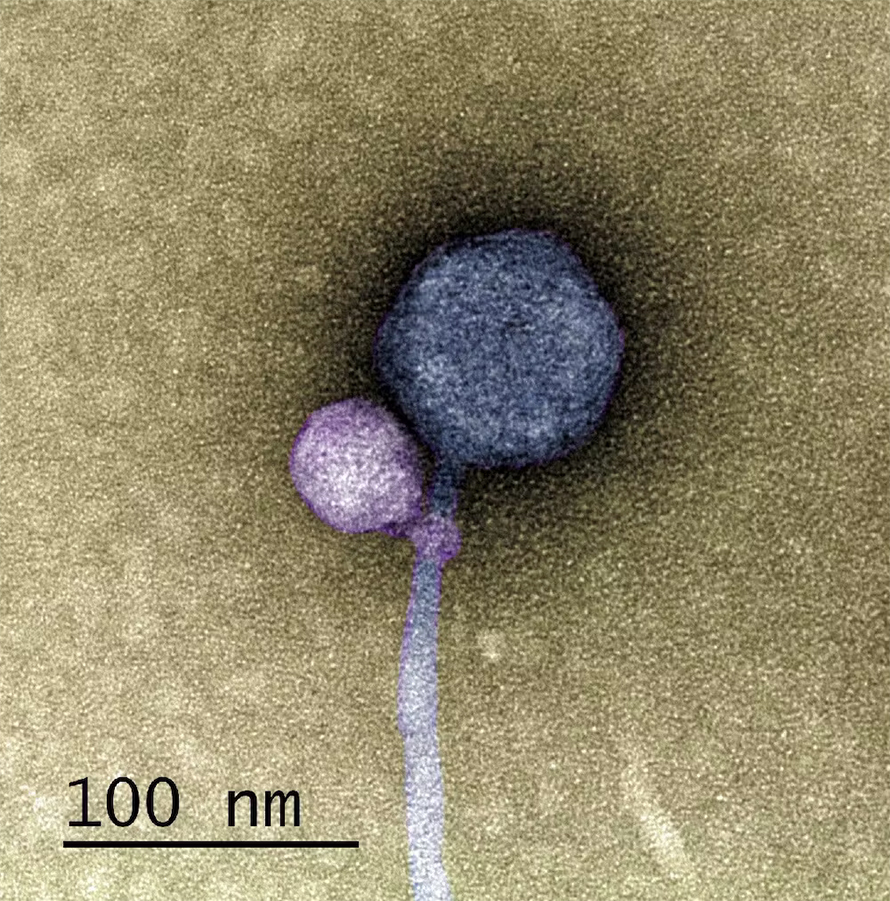
MiniFlayer is the first satellite phage known to have lost its ability to lie dormant. Not being able to lie in wait for your helper to enter the cell poses an important challenge to a satellite phage. If you need another virus to replicate, how do you guarantee that it makes it into the cell around the same time you do?
MiniFlayer addressed this challenge with evolutionary aplomb and horror-movie creativity. Instead of lying in wait, MiniFlayer has gone on the offensive. Borrowing from both “Dracula” and “Alien,” this satellite phage evolved a short appendage that allows it to latch onto its helper’s neck like a vampire. Together, the unwary helper and its passenger travel in search of a new host, where the viral drama will unfold again. We don’t yet know how MiniFlayer subdues its helper, or whether MindFlayer has evolved countermeasures.
If the recent pandemic has taught us anything, it is that our supply of antivirals is rather limited. Research on the complex, intertwined and at times predatory nature of viruses and their satellites, like the ability of MiniFlayer to attach to its helper’s neck, has the potential to open new avenues for antiviral therapy.
This article is republished from The Conversation under a Creative Commons license. Read the original article.
![]()
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

