From the journals: November 2019
We offer a selection of recent papers on a variety of topics from the Journal of Biological Chemistry, the Journal of Lipid Research and Molecular & Cellular Proteomics.
Demystifying mitochondrial morphology in fatty livers
For an ailment nonchalantly dubbed “the unnamed disease” in 1980, nonalcoholic fatty liver disease has become disconcertingly prevalent. NAFLD is now the most common cause of chronic liver disease, affecting between 80 and 100 million people in the United States and close to one billion people worldwide. True to its name, the disease is characterized by a buildup of lipids in the liver; over time, the excess fat can cause inflammation, scarring and, ultimately, organ failure.
Over the past four decades, researchers have been unable to clarify the role of mitochondria in NAFLD — the organelles have been found both to overproduce glucose and to shut down in different patients, changing shape all the while.
To understand whether a change in mitochondrial morphology causes dysfunction or vice versa, researchers at the University of Geneva engineered a mouse lacking in prohibitin-2, a protein in the organelle’s inner membrane that plays a role in cell proliferation, apoptosis and mitochondrial dynamics, or coordinated cycles of fission and fusion.
In a paper published in the Journal of Biological Chemistry, Lingzi Li and colleagues found that knocking out Phb2, which normally forms large ring-shaped complexes with its sibling molecule Phb1, caused the mitochondria to fragment and stop producing glucose. The absence of Phb2 also led to excessive cleavage of OPA1, a protein essential to mitochondrial dynamics.
When the researchers then used adenovirus to express a cleavage-resistant long version of OPA1, L-OPA1 delta, they found that hepatic mitochondria returned to a normal shape due to a stabilization in dynamics but did not regain their previous function of glucose production. When they then expressed L-OPA1 delta in mice that didn’t have Phb2 knocked out, they found it caused excessive mitochondrial respiration and glucose production. Together, their results suggest that cycles of mitochondrial fusion and fission, regulated by prohibitin, play a role in controlling glucose production.
— John Arnst
 Mitochondria often lose their normal rodlike or spherical shapes in the livers of people with nonalcoholic fatty liver disease.David Furness/Wellcome Collection
Mitochondria often lose their normal rodlike or spherical shapes in the livers of people with nonalcoholic fatty liver disease.David Furness/Wellcome Collection
A link between stress and depression
Chronic stress affects the electrophysiology of neurons in various regions of the brain including the medial prefrontal cortex, or mPFC, increasing risk for depression. The mechanisms underpinning these alterations are not understood fully, however. In a recent paper in the Journal of Biological Chemistry, Chenghui Song and colleagues at Scripps Research write that they studied chronically stressed mice to uncover the downstream effectors of GPR158 — a receptor enriched in the mPFC. They found that a complex between GPR158 and RGS7, which is a negative regulator of GPCR signaling, limited potassium current from mPFC neurons in stressed mice, leading to suppressed neuronal excitability. The authors suggest this freshly uncovered mechanism could support the development of new therapies for stress-induced depression.
Using stem cells in veterinary therapy
Stem cells can regenerate themselves and differentiate into various cell types. In recent years, adult stem cells have become important candidates for therapy both in human diseases and in veterinary science, exemplified by the use of bone marrow-derived mesenchymal stem cells, or BMMSCs, to repair locomotor disorders in hard tissues such as bone and cartilage in dogs and cats. However, BMMSCs also can affect the immune system by triggering the release of cell proliferation factors. Thus, researchers need comprehensive understanding of the diversity of BMMSCs in dog breeds to speculate on their biological function.
In a collaborative study published in the journal Molecular & Cellular Proteomics, Filip Humenik and a team of researchers from France and Slovakia used a multi-omics approach to characterize BMMSCs from adult dogs of six breeds and assessed their ability to differentiate into bone, cartilage and fat cells. Conditioned medium derived from these cells showed an angiogenic response in a chicken embryo model, meaning it promoted the formation of new blood vessels from existing blood vessels, which is beneficial for regenerating tissue, but critical for cancer development. This work reinforces the need for more clinical studies before considering MSCs for veterinary treatment.
How liver response changes with age
The glutamine- and asparagine-depleting enzyme asparaginase is used as a treatment for blood cancers, but its clinical success is hindered by side effects including hepatotoxicity. To uncover how age could affect the liver’s response to asparaginase, Inna Nikonorova of Rutgers University and colleagues examined livers from mice of varying ages treated with the enzyme. While juvenile mouse livers experienced steatosis and iron accumulation, adult livers exhibited upregulated serine, glycine and one-carbon metabolism and increased inflammation. These results, recently published in the Journal of Biological Chemistry, suggest that adult and juvenile livers employ different strategies to maintain homeostasis.
A new enemy for drug-resistant cancer cells
Two drugs constitute medicine’s first line of defense against acute myelogenous leukemia: daunorubicin and cytarabine, or Dnr and Ara-C. AML cancer cells quickly grow resistant to these drugs, however, and often require further treatment. Past research has shown that Dnr raises the levels of ceramides, lipids that contribute to apoptosis, in treated cancer cells. Researchers at the East Carolina Diabetes and Obesity Institute recently generated Dnr- and Ara-C-resistant AML cell lines to further investigate ceramide’s role. Their work was published in the Journal of Lipid Research.
Ceramide is broken down to sphingosine, another lipid that can be catabolized further. Li-Pin Kao and researchers in the Cabot lab found that in drug-resistant cell lines, ceramide levels were low and breakdown products were high, indicating cancer cells rewired normal cells’ pathway to evade apoptosis. The enzymes involved in ceramide breakdown had higher activity in resistant cell lines, and ceramide addition no longer affected cancer cells.
Treatment with inhibitors that prevent ceramide breakdown proved beneficial; the researchers saw higher ceramide levels and higher cancer cell apoptosis rates. Higher mitochondrial respiration rates in resistant cell lines were reversed as well. Resistant cancer cells tend to rely on oxidative phosphorylation, which produces more ATP than glycolysis. This dual inhibitor effect on ceramide levels and mitochondrial respiration could help patients with chemoresistant AML.
A deep dive into mesenchymal stem cell differentiation
Human mesenchymal stem cells, or MSCs, are part of our bodies’ connective tissue. They can differentiate into bone, muscle, cartilage and fat cells and are thus beneficial for treating conditions like type 1 diabetes and spinal cord injury and for organ transplantation. However, quite a few challenges are associated with using these cells for therapy. For example, tissue-derived MSCs can trigger an unnecessary immune response, leading to uncontrolled cell proliferation, or can undergo unwanted differentiation. To better control such off-target effects of tissue-derived MSCs, these cells can be differentiated from human embryonic stem cells, or hESCs, in the lab in a more controlled manner. But researchers need to know more about the changes that occur during this differentiation.
To address this, an international team of researchers came together for a multidisciplinary study of hESC-to-MSC differentiation. Led by Anja M. Billing of Weill Cornell Medicine – Qatar, the team combined transcriptomics, proteomics and phosphoproteome analysis for an in-depth understanding of the process. Their findings, recently published in the journal Molecular & Cellular Proteomics, identified cellular pathways and key factors involved in ESC-to-MSC differentiation.
Using RNA sequencing and performing RNA-to-protein correlation, the authors identified specific subsets of genes coding for transcription factors, kinases and phosphatases as well as noncoding RNAs, all of which are differentially expressed in ESCs and MSCs. Using phosphoproteomic analysis, the authors deduced that the proteins that most were most expressed during differentiation also underwent the most extensive phosphorylation, hinting at the biological function of such proteins in this process. Overall, proteins involved in cell-to-cell adhesion, cytoskeleton organization and extracellular matrix formation were the most prominent players driving this differentiation. This study enriches our understanding of the biology of ESC–MSC differentiation, paving the way for better use of MSCs in stem cell–based therapy.
— Isha Dey
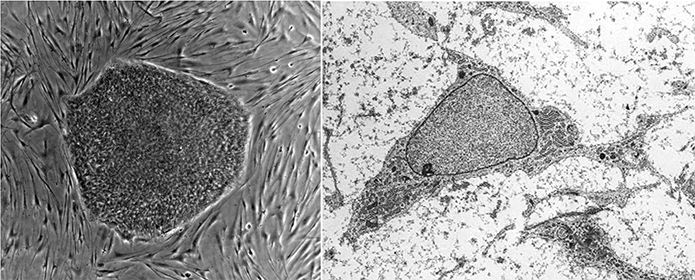 These two images show human embryonic stem cells on mouse fibroblast cell feeder layer (left) and a more highly magnified electron micrograph of a mesenchymal stem cell (right).Wikimedia Commons
These two images show human embryonic stem cells on mouse fibroblast cell feeder layer (left) and a more highly magnified electron micrograph of a mesenchymal stem cell (right).Wikimedia Commons
Disrupting pathogens’ protective biofilms
The fungal pathogen Aspergillus fumigatus causes invasive infections in immunocompromised patients and evades host immune responses by encapsulating itself in a biofilm. A cluster of genes in A. fumigatus recently was linked to the production and modification of key components of the fungal biofilms, galactosaminogalactans, or GAGs. Natalie Bamford of the Hospital for Sick Children in Toronto and colleagues expressed a gene from this cluster in yeast, producing the protein Ega3 from the glycoside hydrolase family 114, or GH114. Functional assays revealed that Ega3 disrupts GAG-dependent biofilms, and structural analysis revealed substrate binding sites on the enzyme. These findings, recently published in the Journal of Biological Chemistry, provide valuable mechanistic insights into the GH114 family, which contains hundreds of members found in plants and human pathogens.
Analyzing diffuse gliomas for personalized medicine
Diffuse gliomas, including astrocytomas, oligodendrogliomas and glioblastomas, are the most common brain tumors. Based on histology and genomic and transcriptomic data such as DNA and RNA copy number and DNA methylation, gliomas broadly are classified as isocitrate dehydrogenase-mutant or IDH–wild-type. Patients with IDH mutations typically live longer, but the mutations are not yet used as therapeutic biomarkers. Ugljesa Djuric and a team in Toronto recently did a proteomic analysis of diffuse gliomas to improve understanding of the downstream pathways involved in their malignancies. Their findings were published in the journal Molecular & Cellular Proteomics.
The authors used mass spectrometry and protein cluster analysis to study more than 5,000 unique proteins in tissue samples from a variety of gliomas. Overall, IDH-mutant gliomas had an increased abundance of proteins involved in mRNA splicing, whereas IDH–wild-type gliomas were enriched in proteins involved in invasiveness and epithelial-to-mesenchymal transition, an important driver of the disease progression. This study sheds light on the changes at the protein level caused by genetic alterations in glioma subtypes, defined by their IDH status, and identifies distinct targetable proteins and pathways, which might aid in developing specific and personalized treatments.
Inactivating a moonlighting enzyme
Tryptophanyl-tRNA synthetase, or WRS, plays key roles in protein synthesis within cells, but the enzyme also performs nonclassical extracellular functions such as the regulation of inflammation. To elucidate how moonlighting WRS is regulated in the extracellular milieu, Parker Jobin of the University of British Columbia and colleagues performed secretion and cleavage assays and proteomic studies of WRS from macrophages. They write in the Journal of Biological Chemistry that they found that WRS increases the activity of matrix metalloproteinases, some of which were shown to cleave WRS at several sites, curbing the proinflammatory signaling initiated by the moonlighting enzyme.
Even more reasons to take your DHA
 DHA, a supplement many already take, could play a role in suppressing melanoma growth.Dawn HaywardThe fatty acids DHA and EPA are popular dietary supplements and are known to slow tumor growth, but how do they do it? Researchers at Iwate Biotechnology Research Center in Japan investigated the effects of docosahexaenoic acid and eicosapentaenoic acid on melanoma cells, uncovering a pathway that fatty acids take to reduce melanin content in tumor cells. If cancer cells no longer can make melanin, the pigment in skin cells, they can undergo apoptosis.
DHA, a supplement many already take, could play a role in suppressing melanoma growth.Dawn HaywardThe fatty acids DHA and EPA are popular dietary supplements and are known to slow tumor growth, but how do they do it? Researchers at Iwate Biotechnology Research Center in Japan investigated the effects of docosahexaenoic acid and eicosapentaenoic acid on melanoma cells, uncovering a pathway that fatty acids take to reduce melanin content in tumor cells. If cancer cells no longer can make melanin, the pigment in skin cells, they can undergo apoptosis.
DHA and EPA are considered omega-3 fatty acids because of their structure. Like saturated fatty acids, omega-3 or unsaturated fatty acids have a hydrophilic head and a long hydrophobic tail, like a snake, but an omega-3’s tail has double bonds, or “kinks,” in certain places. These kinks make it difficult for unsaturated fatty acids to stack on top of one another. Saturated fatty acids, with no kinks, easily can stack and potentially clog arteries.
Hidetoshi Yamada and the research team analyzed melanin content in melanoma cells treated with saturated and unsaturated fatty acids. Unsaturated fatty acids such as DHA and EPA lowered melanin content in the tumor cells. To determine further the role of fatty acids, the researchers looked at melanin synthesis, which involves several steps. They found that EPA reduced the protein levels of an enzyme involved in making melanin in cancer cells, which could contribute to EPA’s anti-tumor effects.
The researchers also found that unsaturated fatty acids prevented synthesized melanin from getting to its destination. Actin, a protein that other molecules can ride on as they move throughout the cell, was reduced in cells treated with EPA. DHA decreased a specific signaling pathway in cancer cells and disrupted their cell cycle.
These findings, which were published in the Journal of Lipid Research, strengthen the argument that increased unsaturated fatty acid intake is beneficial against certain cancers.
— Dawn Hayward
An isolation strategy for cytokines
Cytokines initiate biological activities by binding to and dimerizing receptors. These ligands have garnered interest for use in immunotherapies, but their short half-lives and pleiotropic binding have raised concerns. To improve the stability of cytokine-receptor interactions, Jamie Spangler, Ignacio Moraga and colleagues at Stanford University devised an evolutionary strategy for the isolation of monovalent antibodies that lock receptors into a dimerized state. They developed a stapler that could stabilize the interleukin-4 receptor dimer and another that could stabilize the bond between interleukin-2 and its receptor subunit. These new methods potentially offer a means to modulate the activity of other ligands for a wide scope of therapeutic applications. This study was published in the Journal of Biological Chemistry.
The lipid buildup problem
Our intestinal cells store lipids through carefully regulated pathways. When there are problems, lipids can accumulate, causing inflammation and oxidative stress. To investigate the enzymes that may play a role in this regulation, researchers at the Université de Montréal used genetically modified intestinal cell lines to find out what happens when an important GTPase (an enzyme involved in breaking down GTP) called Sar1b is knocked out. Sar1b is involved with transporting fat-carrying molecules called chylomicrons and associated with certain intestinal lipid diseases. Their findings were published in the Journal of Lipid Research.
Alain Sane and the research team looked at oxidative stress, inflammation and fatty acid oxidation to determine the effects of losing the GTPase protein. They found a reduction in antioxidant protection by measuring levels of glutathione along with a decrease in redox homeostasis regulation. Inflammation markers also were increased in response to GTPase knockout. The researchers then looked at fatty acid synthesis and breakdown. Breakdown was decreased but synthesis increased in cells with GTPase knockout, indicating a disruption in homeostasis. GTPase function is therefore critical to preventing lipid accumulation and inflammation that can occur in chylomicron retention disease.
Solving structure of useful enzymes
Xyloglucans, or XyGs, are glycans contained in the cell walls of most land plants. Because of their potential applications in various industrial sectors, enzymes that can alter or degrade these glycans are sought after. The GH74 family of enzymes has demonstrated a specificity for XyG, but there have been conflicting reports of their structures and modes of action. In a paper in the Journal of Biological Chemistry, Gregory Arnal of the University of British Columbia and colleagues write that they performed an in-depth biochemical characterization of several proteins, solving the crystal structures of four GH74 enzymes. The study unveiled critical structure-function relationships that help refine our understanding of GH74 catalysis.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.




