JBC: Targeting semen amyloid fibrils to reduce HIV infectivity
The human immunodeficiency virus, which attacks the immune system, affects more than 1.2 million people in the U.S. There aren’t any vaccines or cures. Instead, microbicides are used to help protect against the transmission of HIV from person to person. However, the process of transmission isn’t understood fully and can involve both viral and human factors that promote infection.
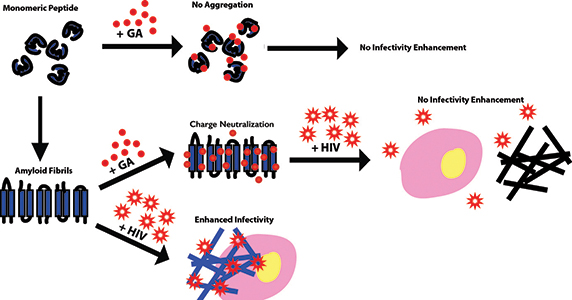 Gallic acid, or GA, coats the surfaces of amyloid fibrils in semen to prevent HIV infectivity enhancement and coats the peptide precursors to prevent fibril formation.IMAGE COURTESY OF JOSIE LORICCO
Gallic acid, or GA, coats the surfaces of amyloid fibrils in semen to prevent HIV infectivity enhancement and coats the peptide precursors to prevent fibril formation.IMAGE COURTESY OF JOSIE LORICCO
In a recent paper published in the Journal of Biological Chemistry, Nadia Roan of the University of California, San Francisco, and George Makhatadze of Rensselaer Polytechnic Institute described a small molecule that prevents a specific human factor from increasing the ability of HIV to cause infection.
Researchers know that the virus itself has many factors that help it infect new hosts. There are also human factors that play a role in the transmission of HIV and a person’s susceptibility to infection. One of these factors is the ordered accumulations of misfolded proteins called amyloid fibrils. These fibrils occur naturally in human semen and have been shown to increase HIV infectivity and decrease the effectiveness of anti-HIV microbicide treatments.
The infection-promoting fibrils have been observed in the semen of both healthy and HIV-infected men. Therefore, researchers want to identify compounds that disrupt the formation of these fibrils or rid them of their infectivity-enhancing properties and reduce the sexual transmission of the virus through semen.
The investigators, led by graduate student Josie LoRicco of Rensselaer Polytechnic Institute, used a screen of small molecules to identify compounds that altered the properties of specific amyloid fibrils in semen. One molecule that came out of the screen, gallic acid, further proved to be capable of reducing HIV infectivity in the presence of semen. “Gallic acid is a small molecule found naturally in many foods, including grapes and tea,” says Makhatadze.
LoRicco, Roan, Makhatadze and colleagues further investigated gallic acid’s properties. They used atomic force and confocal microscopies in addition to several quantitative assays to characterize the interaction between gallic acid and the fibrils. Surprisingly, gallic acid did not induce disassembly of the fibrils but instead bound to their surfaces.
The investigators conducted biophysical analysis of fibrils’ surface properties to understand the nature of the interaction. They demonstrated that gallic acid limits the ability of semen fibrils to enhance HIV infection by binding to the fibrils’ surfaces and neutralizing their surface charge. Additionally, the gallic acid-coated fibrils prevent the formation of new amyloid fibrils by binding the precursor components and changing their charge characteristics.
“Gallic acid appears to do two things,” explains Makhatadze. “First, it inhibits new fibril formation. Second, it interacts with pre-existing fibrils and renders them incapable of facilitating HIV infectivity.”
The investigators suggest that gallic acid may be a useful addition to multicomponent microbicides that target both viral and human factors involved in the promotion of HIV transmission and infection. Makhatadze suggests that “such combination microbicides will be more effective at preventing transmission compared to single-component microbicides.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

