From the journals: September 2018
We offer a selection of recent papers on a variety of topics from the Journal of Biological Chemistry, the Journal of Lipid Research and Molecular & Cellular Proteomics.
Fat metabolism is linked to sleeping disorder
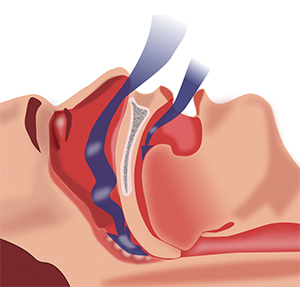 This diagram shows the obstruction of ventilation in sleep apnea.Courtesy of Habib M’henni/Wikimedia Commons Obstructive sleep apnea, or OSA, is a clinical disorder characterized by recurring partial or complete blockage of the upper respiratory airway during sleep, causing the patient to wake up abruptly, gasping and choking. A 2014 report by the National Healthy Sleep Awareness Project states that at least 25 million adults in the U.S. suffer from obstructive sleep apnea. Another study shows that prevalence of OSA in the U.S. has increased over the past two decades, owing to an increase in obesity cases. This, along with the fact that OSA is associated with high blood pressure and stroke, indicates a possible link between OSA and lipid levels in the human body.
This diagram shows the obstruction of ventilation in sleep apnea.Courtesy of Habib M’henni/Wikimedia Commons Obstructive sleep apnea, or OSA, is a clinical disorder characterized by recurring partial or complete blockage of the upper respiratory airway during sleep, causing the patient to wake up abruptly, gasping and choking. A 2014 report by the National Healthy Sleep Awareness Project states that at least 25 million adults in the U.S. suffer from obstructive sleep apnea. Another study shows that prevalence of OSA in the U.S. has increased over the past two decades, owing to an increase in obesity cases. This, along with the fact that OSA is associated with high blood pressure and stroke, indicates a possible link between OSA and lipid levels in the human body.
A patient-based study led by Luciano Drager at the University of Sao Paolo, Brazil, and reported in the Journal of Lipid Research provides insight into the effect of OSA on lipid metabolism and a possible mechanism by which continuous positive airway pressure, or CPAP, a special ventilator to continuously keep airways open, works to treat OSA patients. In this study, OSA patients were found to have reduced breakdown of triglyceride-rich lipoproteins and, subsequently, reduced clearance of their metabolites from the plasma compared to non-OSA control individuals. Treating OSA patients with CPAP significantly improved plasma lipid clearance by increasing lipolysis of the triglycerides.
OSA leads to many cardiovascular complications including stroke, mainly due to fat deposits along the inner arterial walls, making them thicker. This study provides an understanding of a possible mechanism behind this. Since OSA also increases insulin resistance and is associated with the development of type 2 diabetes, a major global health problem, and type 2 diabetes patients have an increased risk of heart disease, this study provides insight into the physiology of how disease symptoms and causes are interconnected.
— Isha Dey
A protein’s role in oxidative stress in obese mothers
Excessive body fat and obesity have been associated with negative effects on female fertility and pregnancy. In mice, maternal obesity impairs proper development of egg precursors called oocytes and results in oxidative stress. In a recent paper published in Molecular & Cellular Proteomics, Qiang Wang and colleagues at the State Key Laboratory of Reproductive Medicine in China describe the link between poor oocyte development and oxidative stress.
The authors used proteomics to compare obese and nonobese mother mice and found that the protein TP53-inducible glycolysis and apoptosis regulator, or TIGAR, had reduced expression in the mice with a high-fat diet. Reactive oxygen species, or ROS, were increased when TIGAR was depleted in mouse oocytes, which led to errors in downstream cell division events and strongly activated autophagy. Overexpression of TIGAR partly corrected cell division errors, decreased ROS production and reduced autophagy levels. Wang and colleagues further showed that the ROS production controlled by TIGAR is the driving force for the downstream autophagic response, thereby providing a link between TIGAR-mediated redox homeostasis and cell development. This study provides evidence of the direct effects of maternal obesity on the quality of oocyte development and implicates TIGAR in oocyte maturation in mice, which may have implications for how pregnancies are monitored for obese mothers.
Redox sensing in the mosquito gut
Mosquitoes vary in their ability to carry diseases and also in their insecticide resistance; understanding this variation is key to developing vector control strategies. Gabriela O. Paiva-Silva and colleagues at the Instituto Nacional de Ciência e Tecnologia em Entomologia Molecular in Brazil demonstrated that the redox-sensitive transcription factor nuclear factor erythroid 2-related factor 2, or Nrf2, coordinates multiple stress responses in the midgut of the mosquito Aedes aegypti. Nrf2 depletion affected intestinal stem cell homeostasis, redox balance, viral load and responses to insecticide challenge. Thus, the Nrf2 gene could be a candidate for genetic modification to decrease the transmission of viruses such as Zika. The research was published in the Journal of Biological Chemistry.
The road to better treating drug-resistant TB
 This scanning electron micrograph shows Mycobacterium tuberculosis bacteria, which cause tuberculosis.NIAID Tuberculosis is a bacterial infection caused by Mycobacterium tuberculosis. It is the leading cause of infectious-disease death worldwide. Global efforts to control TB infection have been hindered by the rise of drug-resistant strains. Specifically, resistance against isoniazid, one of the first-line drugs to treat those infected with TB, has emerged rapidly and has expanded among Mtb strains across the globe.
This scanning electron micrograph shows Mycobacterium tuberculosis bacteria, which cause tuberculosis.NIAID Tuberculosis is a bacterial infection caused by Mycobacterium tuberculosis. It is the leading cause of infectious-disease death worldwide. Global efforts to control TB infection have been hindered by the rise of drug-resistant strains. Specifically, resistance against isoniazid, one of the first-line drugs to treat those infected with TB, has emerged rapidly and has expanded among Mtb strains across the globe.
A recent paper by Luisa Nieto Ramirez of Colorado State University and colleagues published in Molecular & Cellular Proteomics helps improve our understanding of the physiological consequences of resistance for bacterial populations by using proteomic and metabolic comparisons of strains with or without isoniazid resistance. The researchers used clonal pairs, meaning the pairs originated from the same genetic lineage with the only difference being a known genetic mutation that made one of the clones resistant to isoniazid. Two types of pairs were used; one set came from a laboratory-adapted strain, and the other came from a clinical strain.
The group looked at differences between isoniazid-sensitive and -resistant clonal pairs and found that the pair from the clinical strain background showed more changes than the pair from the laboratory-adapted background. Therefore, they concluded that genetic background does affect the proteomic changes that occur after isoniazid resistance is acquired, suggesting that a one-size-fits-all treatment strategy may not work across resistant strains from different sources. They also identified 26 proteins that changed in abundance in both types of resistance clones, indicating that some proteomic rearrangement events are independent of genetic background. Many of these proteins were implicated in energy metabolism, beta-oxidation and alternative lipid biosynthesis. These shared changes could be exploited as novel targets for drug development to combat isoniazid resistance and improve treatment strategies.
— Courtney Chandler
Long RNA goes wrong in kidneys
Autosomal dominant polycystic kidney disease is one of the most common monogenic diseases; kidneys develop fluid-filled cysts that can eventually lead to kidney failure. Peter Igarashi and colleagues at the University of Minnesota investigated the roles of long noncoding RNAs, or lncRNA, in the pathogenesis of the disease. They found a kidney-specific lncRNA that was downregulated in a mouse model of the disease and in cystic kidneys from patients. Deleting this lncRNA in cells increased mTOR signaling and mitochondrial respiration, suggesting that these are pathways to investigate to understand the disease. The research was published in the Journal of Biological Chemistry.
Gangliosides control obesity, coat color
Diabetes and obesity are two of the most serious clinical concerns in the U.S. A 2010 report showed 32.2 percent of men and 35.5 percent of women are obese, and type 2 diabetes affects 27 million to 29 million Americans with signs of dyslipidemia, indicating a relationship between diabetes and lipid metabolism.
Gangliosides are sialic acid-containing glycosphingolipids that play important roles in cell signaling. The ganglioside GM3, a precursor for making complex gangliosides, is important in development and disease. GM3 deficiency leads to reduced brain development and function, whereas GM3 is elevated in the fat cells of obese animals and in the plasma of type 2 diabetic patients, inducing insulin resistance. A recent study by Kei-ichiro Inamori and a team from Tohoku University and Nippon Boehringer Ingelheim Co. in Japan, and published in the Journal of Lipid Research has singled out GM3 synthase, or GM3S, as a critical factor in controlling obesity and insulin resistance. When genetically obese mice were modified to lack GM3S, they showed reduced body weight and improved insulin and glucose tolerance. Also, preliminary observations showed that GM3S knockout mice had a different coat color than the unmodified obese ones.
This finding provides evidence for the contribution of lipid metabolism to obesity and diabetes. Further studies with melanin synthesis would give a better understanding of the role of lipid metabolism in skin color development.
Bitter signals from pathogens
Bitter taste receptors are not found only on the tongue; these G-protein–coupled receptors can be found in diverse tissues, including the airways. In a study published in the Journal of Biological Chemistry, Robert J. Lee and colleagues at the University of Pennsylvania examined the non-taste-related functions of bitter taste receptors by studying receptors expressed in the cilia of cells in the nose and sinuses. In response to quorum-sensing molecules produced by the common airway pathogen Pseudomonas aeruginosa, the receptors were activated and participated in signaling pathways important to innate immunity.
Cell-replacement therapy for Parkinson’s disease
Cell-replacement therapy shows promise as a treatment for neurodegenerative diseases such as Parkinson’s disease. The therapy for Parkinson’s involves differentiating stem cells into dopaminergic neurons and transplanting them into patients. However, transplantation outcomes are affected by variability in differentiated cells, including contamination with other neuronal cell types or residual undifferentiated stem cells.
A paper in Molecular & Cellular Proteomics describes an isolation procedure that relies on novel cell surface markers to yield a more homogenous population of differentiated neurons. Hossein Baharvand, Ghasem Hosseini Salekdeh and their international team developed a transgenic human embryonic stem cell line that contains a green fluorescent protein reporter under control of a transcription factor involved in dopaminergic neuronal development. They tracked differentiation using GFP expression and used quantitative proteomics to identify proteins that were enriched in partially differentiated cells called dopaminergic progenitors. Using antibodies against one of these proteins, called contactin 2, they isolated progenitors and transplanted them into rats modeling Parkinson’s. Rats treated with contactin 2-enriched cells had enhanced dopamine release and reduced Parkinson’s phenotypes compared with rats treated with unsorted cells. This work provides a strategy to improve transplantation success by better selecting for dopaminergic progenitor cells, representing an important step toward standardizing cell-replacement therapy.
Tau, synuclein and amyloid use different doors
Misfolded aggregates of the proteins tau, alpha-synuclein or beta-amyloid spread from cell to cell during the progression of Alzheimer’s and Parkinson’s diseases. Binding of these aggregates to heparan sulfate proteoglycans, or HSPGs, on cell surfaces triggers their intake into cells. Marc Diamond of the University of Texas Southwestern and an international team examined how sulfation and glycation patterns of HSPGs affected their interactions with these protein aggregates. Tau aggregates required a very specific glycosaminoglycation and sulfation pattern for binding and cellular uptake, whereas alpha-synuclein and beta-amyloid interactions with HSPGs were more complex and variable. The research was published in the Journal of Biological Chemistry.
Smoothening heart rhythms
The G-protein–coupled receptor Smoothened, or SMO for short, has a central, canonical role in the Hedgehog developmental signaling pathway in vertebrates. Natalia A. Riobo–Del Galdo and colleagues at Thomas Jefferson University investigated SMO’s role in a noncanonical Hedgehog signaling pathway in cardiomyocytes and intact hearts. They found that SMO activation selectively controlled outward voltage-gated potassium repolarizing currents; an SMO agonist induced ventricular arrhythmias. The noncanonical Hedgehog signaling pathway may thus be important for understanding heart health. The research was published in the Journal of Biological Chemistry.
Screening heart disease drugs 10,000 times faster
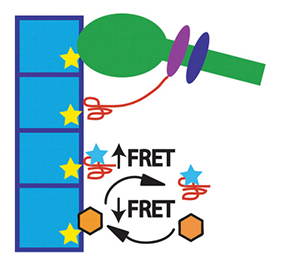 This cartoon diagram showing the FRET assay developed by David Thomas’ lab accompanied a commentary on Thomas’ JBC paper. The method was used to screen hundreds of compounds for interactions with actin.Courtesy of Laura K. Gunther and Christopher M. Yengo Actin and myosin are an iconic duo. The pair of filamentous proteins grip and slide past one another, generating the force that allows muscles to contract and cells to move and divide. But this dynamic relationship can go wrong: When interactions between actin and myosin are either too weak or too strong, muscle or heart disease can result.
This cartoon diagram showing the FRET assay developed by David Thomas’ lab accompanied a commentary on Thomas’ JBC paper. The method was used to screen hundreds of compounds for interactions with actin.Courtesy of Laura K. Gunther and Christopher M. Yengo Actin and myosin are an iconic duo. The pair of filamentous proteins grip and slide past one another, generating the force that allows muscles to contract and cells to move and divide. But this dynamic relationship can go wrong: When interactions between actin and myosin are either too weak or too strong, muscle or heart disease can result.
Researchers at the University of Minnesota overseen by muscle researcher David Thomas developed a method to quickly screen thousands of chemical compounds to evaluate their effect on actin-myosin binding. The results were published in the Journal of Biological Chemistry.
The method is based on fluorescence resonance energy transfer technology, or FRET, which is used to sense interactions between molecules by detecting the transfer of energy between a fluorescent molecule and a nonfluorescent sensor molecule. Thomas’s team improved on the method to make it faster and more precise.
“We call it direct waveform recording, and it’s 10,000 times faster than the standard way to measure fluorescence lifetimes,” Thomas said. “As a result, we can use fluorescence lifetime detection for high-throughput applications such as the screening of large chemical libraries for drug discovery.”
Piyali Guhathakurta, a research associate in Thomas’ lab, led the effort to screen compounds that affect how myosin and actin interact. Out of 727 compounds tested, 10 significantly affected actin-myosin binding. These compounds now can be tested further for potential clinical applications.
Previously, only two potential drugs targeting actin-myosin interactions have entered clinical trials. By accelerating the early stages of chemical screening, the researchers hope to facilitate faster discovery of heart and muscle disease drugs. Thomas has started a company, Photonic Pharma, out of his lab to commercialize and license the compounds discovered using these methods.
“It opens up a lot of possibilities that were not known to be worth pursuing before,” Thomas said.
— Sasha Mushegian
DAG dictates correct cell division
In cell division, or mitosis, one cell undergoes multiple regulated steps to form two daughter cells. Essentially, the process consists of doubling chromosomes and disrupting the nuclear membrane followed by separating chromosomes to opposite ends of the cells, reassembling the nuclear envelope and, finally, dividing the cytoplasm of the parental cell to form the two similar cells. Disruption in nuclear membrane reassembly leads to genome instabilities in cancer, which makes nuclear membrane reassembly an important topic in cancer research. Diacylglycerol, or DAG, a downstream molecule of G-protein−coupled receptor signaling, has been shown to be essential in forming the nuclear envelope in nonsomatic cells.
A recent collaborative study by Gary Chung and a team in the United Kingdom and the U.S., published in the Journal of Lipid Research, has identified the novel role of DAG in regulating nuclear envelope reassembly during mitosis in somatic cells. The authors found DAG to be localized to the reforming nuclear envelope. Moreover, depleting DAG from cis Golgi, its cellular reservoir, reduced the rim curvature of the nuclear envelope, which is important for pore formation and correct development of the nuclear membrane. This finding opens up new avenues of research with respect to the role of lipids in controlling cell division and thus cancer.
The power of bicelles
Methanotrophic bacteria convert methane to methanol, so they are a promising source of enzymes for methane remediation and biofuel production. However, key enzymes from these bacteria, such as particulate methane monooxygenase, or pMMO, lose activity when removed from membranes or reconstituted in detergent micelles, making them difficult to study or adapt for these practical applications. In a study in the Journal of Biological Chemistry, Amy Rosenzweig and colleagues at Northwestern University reconstituted pMMO into bicelles, detergent-embedded discoidal lipid bilayers. Expressing the enzymes in this form restored methane oxidation activity and enabled the pMMO crystal structure to be determined. These results showcase the benefits of reconstituting membrane proteins in bicelles or other membranelike environments.
Hacking hairpin heme
Natural heme-binding proteins are primarily helical, and engineered heme-binding proteins have mostly been based on helical scaffolds. Nagasuma Chandra and colleagues at the Indian Institute of Science explored alternative architectures by designing a water-soluble heme-binding peptide with a beta-hairpin conformation. In their report in the Journal of Biological Chemistry, the authors propose evolutionary explanations for the absence of this topology in natural heme-binding proteins and suggest that the peptide could be used in applications targeting heme.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.



.jpg?lang=en-US&width=300&height=300&ext=.jpg)