Great hope for immunotherapy
In the late 1800s, William B. Coley created a concoction out of bacteria and injected it into cancer patients. The first patient treated with what became known as “Coley’s Toxins” — a 21-year-old man with an inoperable tumor — was cured of his cancer. Though that might not have been the very first foray into immunotherapy as cancer treatment, it certainly was one of the earliest. Coley spent decades studying how bacterial infections affected cancers, earning him the moniker of the “father of immunotherapy.” Since then, the field has come a long way.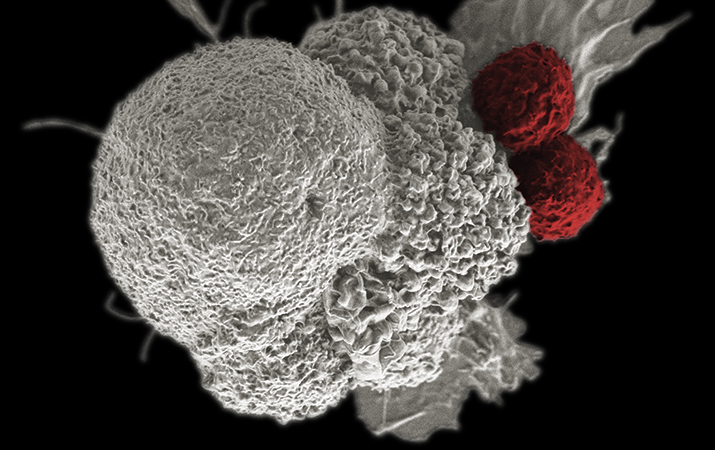 Two T cells attack a cancer cell.NATIONAL INSTITUTES OF HEALTH
Two T cells attack a cancer cell.NATIONAL INSTITUTES OF HEALTH
Immunotherapy is a means of encouraging a patient’s own immune defense mechanisms to do what they’re already supposed to — protect the body against bad stuff. Immunotherapy researchers and practitioners have had a recent spate of dramatic successes in the area of cancer treatment. Some members of the public have taken note, and the recent announcements of two new immunotherapy centers focused on cancer research and treatment — the Parker Institute for Cancer Immunotherapy and the Bloomberg–Kimmel Institute for Cancer Immunotherapy — highlight a newfound, popular appreciation for the field.
But why has immunotherapy suddenly become such a darling of cancer research and its funders?
The answer lies in its potential to be a broadly used and durable treatment.
Evasive maneuvers
Almost all cells in the body carry the same DNA and are capable of performing every cellular function. But they don’t. Intricate mechanisms regulate cells to ensure each performs only its assigned function.
But cancer cells are shifty. Tough to target, they can activate mechanisms beyond their original function. This way, they can avoid the processes that normally keep them in check.
In a process called immune evasion, cancer cells can hijack the cellular mechanisms of immune tolerance and immune exhaustion. In these mechanisms, the body’s T cells learn to recognize the types of signals they should respond to and those they should overlook. Inhibitory receptors on the surface of T cells are a part of immune tolerance and exhaustion. When one T cell wants to let another know it shouldn’t be attacked, it displays ligands on its surface that are recognized by the inhibitory receptors of the T cell. When they see these ligands, the T cells put on the brakes.
When this target is on a cancer cell, the cancer is free to continue living and growing inside the body, avoiding the surveillance of the immune system and achieving immune evasion.
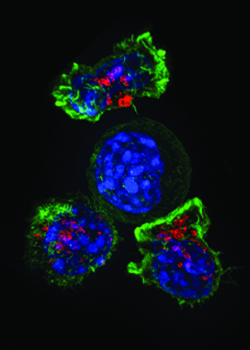 A group of T cells (green and red) surround a cancercell (blue, center).IMAGE BY ALEX RITTER, JENNIFER LIPPINCOTT SCHWARTZ AND GILLIANGRIFFITHS, NATIONAL INSTITUTES OF HEALTH
A group of T cells (green and red) surround a cancercell (blue, center).IMAGE BY ALEX RITTER, JENNIFER LIPPINCOTT SCHWARTZ AND GILLIANGRIFFITHS, NATIONAL INSTITUTES OF HEALTH
Unbraking the system
Immunotherapy aims to counter the tricks cancers use to persist in the body. For example, Herceptin, an antibody that targets a disease-specific pathway in certain breast cancers, is a form of immunotherapy.
Other cell-based immunotherapies, like engineered T cells, allow scientists manually to construct cells that can target each patient’s specific cancer. These types of therapies have the potential to treat many patients but are limited by current technologies and the cost.
Enter a focus on therapies that work against immune checkpoint molecules. These agents, which could have treatment potential for a number of diseases, are antibodies designed to release the brakes on the broken immune-tolerance system of which cancer cells often take advantage. The antibodies interfere with the interactions of inhibitory receptors on T cells and their corresponding ligands, effectively alleviating the block that keeps the T cells from responding.
These antibodies work against specific inhibitory receptors on T cells called PD-1 (or its associated binding partner, PDL-1) and CTLA-4. First made popular in clinical trials for the treatment of melanoma, these antibodies over the past few years have been used to treat many other cancer types, and the patient recovery data have been striking. Patients have responded with long-lasting results in some cancers, including those cancers resistant to other treatments, such as Merkel cell carcinoma, melanoma and non-small cell lung cancer.
The reason for the antibodies’ generalizability is simple: PD-1 and CTLA-4 are molecules every person has within his or her body. They aren’t individualized therapies like many of the cell-based vaccine strategies that require tailoring in the lab.
“What’s exciting about this is it doesn’t involve cell therapies,” says Melody Swartz at the University of Chicago. “It’s just something you inject; it’s just an antibody.”
Which brings up a critical point with these types of treatments: Researchers are unleashing the body’s own exquisitely specific response against cancer.
Unfortunately, though they are potentially generalizable, the strategies currently work in only a subset of patients. Paul Nghiem at the University of Washington Medical School and the Fred Hutchison Cancer Research Center points out that while early results may be encouraging, the therapies targeting PD-1 and CTLA-4 are “dumb.” He explains, “We’re really just releasing the brakes on this smart system, and we’re not smart enough to understand what the system is seeing yet.”
In cases where patients don’t see lasting improvements with PD-1 and CTLA-4-based treatments, researchers believe it’s likely that they haven’t formed a significant response to their own cancer before the treatments are administered or their cancer has found an alternate method of side-stepping immune regulation. Determining which patients will respond to these therapies and which will not is an ongoing area of investigation.
Holding the spotlight
Research topics go in and out of fashion. One day everyone is shouting about stem cells, and the next they are excited about the microbiome. Some believe that immunotherapy has the potential to stay in the spotlight.
To Nghiem, immunotherapy’s staying power can be found in the results of numerous clinical trials. Like the important cancer strategies that came before it — surgery, radiation and chemotherapy — it will stick around in an oncologist’s treatment repertoire because it does work for many people. “I believe — and I’m certainly not alone — that this is now absolutely clearly planted as one of our pillars of therapy for cancer,” says Nghiem.
David Kaufman, executive director of oncology clinical research for Merck Research Laboratories, agrees. “For patients who do have a response to immunotherapy, the benefit is extremely durable,” he says. “We haven’t seen that kind of durability of response with the vast majority of other anti-cancer agents out there.”
Indeed, immunotherapies — and especially those targeting the immune checkpoint blockades — offer some of the most generally applicable treatments seen in cancer therapy in a while. Cancer types in which the immunotherapy Merck drug Keytruda (an anti-PD-1 antibody) has shown clinical promise are in the double digits, says Kaufman. That number likely will increase as more clinical trials emerge.
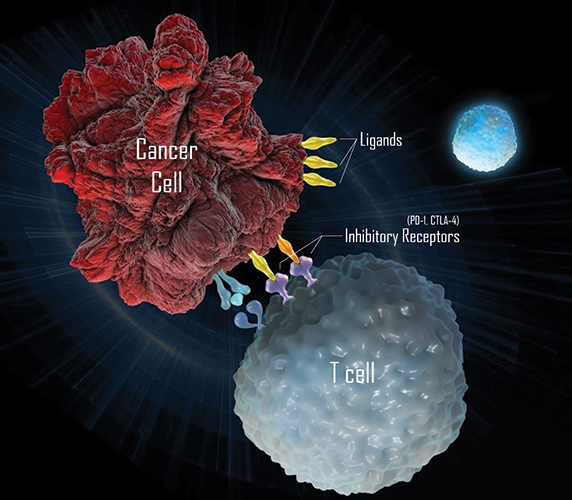 Immunotherapies can use antibodies against inhibitory receptors on T cells.MERCK
Immunotherapies can use antibodies against inhibitory receptors on T cells.MERCK
More to learn
Though the field of immunotherapy is booming, researchers have a lot to learn. Most of the current clinical trials, though promising, still work in only a few patients. Moreover, the trials are still too new to observe long-term consequences.
There also may be reasons to be cautious about the potential long-term effects of checkpoint inhibitors. Their action is broad, taking the brakes off all immune cells, not just those that target cancer cells. This could lead to autoimmune side effects, as were seen in early clinical trials of anti-CTLA-4 agents. Beyond that, these checkpoint inhibitors likely serve other functions within the body that scientists don’t fully understand yet, like their involvement with developing immune memory.
“The immune system is really complicated and highly regulated,” warns Swartz, who also is interested in the process of immune regulation and memory. “Checkpoint inhibitors have a lot of promise, but only if we really understand the broader consequences.”
The unknown intricacies of the immune system were highlighted in a big way with the closing of a recent clinical trial targeting acute lymphoblastic leukemia. The trial was using engineered T cells as a therapeutic against the disease but had to close after three patients died of excess fluid in their brains. The company responsible for the trial, Juno Therapeutics, is known for their work using immunotherapies to treat cancer. Though these devastating side effects might have been a result of the T cell therapy itself, Juno suspects the problems had more to do with the addition of another drug to the trial, which hadn’t been used previously. Clearly, more research is needed to thoroughly understand how these treatments work. And that’s where recent large-scale donations can have a big impact.
The future of immunotherapy
Sean Parker, former Facebook president and co-founder of the music sharing site Napster, has promised $250 million to create the Parker Institute for Cancer Immunotherapy. Philanthropist Sidney Kimmel and former New York City mayor Michael Bloomberg, along with more than a dozen other supporters, will donate $125 million to fund the new Bloomberg–Kimmel Institute for Cancer Immunotherapy. And both the centers have touted collaboration and streamlining clinical research as major facets of their research plans.
Scientists certainly were collaborating before these large centers started appearing. However, the process of making agreements between pharmaceutical companies, research institutions and clinical centers from a diverse range of locations was tedious, to say the least.
Nghiem, who led clinical trials using Keytruda in Merkel cell carcinoma patients, knows the difficulties of cooperating firsthand and understands how meaningful streamlining the collaborative process in research can be. He says, “If these centers can facilitate those communications and break down those barriers and silos, that’s really a key role they can play.”
Past is prologue
The immunotherapies of today are nothing like “Coley’s Toxins,” which, by the way, received their fair share of criticism over the years. But there is one parallel worth noting, and that is the promise of philanthropy.
In October 1890, a year before he reported the first results from his bacterial vaccine experiments, the then-28-year-old surgeon Coley took on a new patient. Bessie Dashiell had a bump on her hand after pinching it between the seats of a Pullman car.
Coley at first suspected Dashiell just had a serious bruise, but her pain worsened. Ultimately, after consulting other experts, he determined that Dashiell had sarcoma. She agreed to an amputation at the elbow. But the cancer had spread, and she died a few months later.
One of Dashiell’s very good friends was John D. Rockefeller Jr., son of the founder of Standard Oil. In his 1998 book “A Commotion in the Blood: Life, Death and the Immune System,” Stephen S. Hall described how Dashiell’s death influenced Rockefeller’s giving philosophy:
“As a young adult, he dedicated much of his philanthropic effort to the conquest of cancer; those efforts began five years after Dashiell’s death, in 1896, with dabbling support for William Coley’s research …, grew prodigiously with his family’s creation of the Rockefeller Institute for Medical Research (now Rockefeller University), and led ultimately to a multimillion-dollar gift that allowed creation of Memorial Hospital (now Memorial Sloan-Kettering Cancer Center) at its present site in New York City. Asked many years later how he became interested in cancer research, Rockefeller replied, ‘I think it goes back to Bessie Dashiell … Her death came to me as a great shock.’”
According to a brief biography of Coley written by Edward F. McCarthy in 2006 in the Iowa Orthopaedic Journal, Coley also received in 1902 part of a large grant from the Huntington family for cancer researchers. “This endowment was the first in the United States designated specifically to study cancer,” McCarthy wrote.
Relatively speaking, these recent donations aren’t huge. When you take into account the billions of dollars spent in any given year on scientific research, these donations can feel like a drop in the bucket.
But some researchers believe focusing on the most clinically relevant research is a smart move. “These initiatives have very thoughtfully targeted this money to the translational research interface, which is where I think donations of that size can really make a significant impact,” says Kaufman.
The centers also draw public attention to the immunotherapy research arena. While some might worry that these high-profile funds might put too much pressure on scientists to find a cure quickly, others remain cautiously optimistic. Even small donations can make big differences when they’re used efficiently, and big-name donations might encourage nonscientists to lend their support. As Nghiem says, “Success and attention and giving from big donors will probably even raise all the boats, rather than deflate people’s enthusiasm and willingness to support cancer research.”
Though Coley’s concoctions went in and out of vogue, it’s clear that he was a man ahead of his time. And even if the investments by Parker, Kimmel and Bloomberg don’t deliver results as striking as are hoped, perhaps one day a future generation will look back and appreciate that they helped get the ball rolling — again.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

