The science behind kratom’s strange leaves
Step into the forest around a rural Malaysian village, pluck the red-, green- or white-veined leaves off a kratom tree, and start chewing. At a small dose, the leaves will act as a stimulant helpful for getting through long hours of hard labor. At a much higher dose, they may cause nausea, vomiting and indigestion, followed by euphoria. At a dose somewhere in between, kratom becomes a highly effective painkiller that can take the edge off after a day’s work. It also can stave off the symptoms of heroin withdrawal.
The leaves of Mitragyna speciosa, kratom’s Latin name, have been used for centuries throughout Southeast Asia for all of the aforementioned properties. The plants grow wild in Malaysia, Thailand and Indonesia, with related species of the genus Mitragyna ranging as far as India and South Africa.

In the United States, kratom use has surged in the past decade amid the ongoing opioid crisis, which claims dozens of lives each day.
Last fall, the U.S. Drug Enforcement Administration proposed classifying kratom as a schedule I drug. Drugs in that category have no approved medical use, and it is extremely onerous to obtain approval for research on them.
The American Kratom Association, the largest kratom advocacy group in the United States, helped spur an outpouring of public comments. In response, the DEA put a hold on its scheduling plans, and the Food and Drug Administration now is preparing an eight-factor analysis of kratom’s safety.
In the meantime, research exploring the interactions of kratom’s components with mice and molecular receptors putters on.
Opioid effects
 A mitre made in 1724 by Abraham of Caesarea (present-day Kayseri, Turkey) and presented to the Monastery of the Virgin Mary near Ankara by the city’s Armenian bishop Moses. Kratom gets its genus name, Mitragyna, from the leaves’ resemblance to these ceremonial hats.Walters Art Museum
A mitre made in 1724 by Abraham of Caesarea (present-day Kayseri, Turkey) and presented to the Monastery of the Virgin Mary near Ankara by the city’s Armenian bishop Moses. Kratom gets its genus name, Mitragyna, from the leaves’ resemblance to these ceremonial hats.Walters Art Museum
The earliest references to kratom’s use as an opium substitute in Western medical literature appeared in 1836, but it has been used for various purposes in Southeast Asia for centuries.
Mitragyna speciosa belongs to the same family, Rubiaceae, as coffee plants and gets its genus name from the leaves’ resemblance to a bishop’s ceremonial hat, called a miter.
The leaves from kratom trees contain at least 37 different alkaloids, a class of nitrogen-atom-containing compounds, but they have two primary active components: mitragynine and 7-hydroxymitragynine, or 7-OHMG, an oxidized analog of mitragynine.
The term “opiate” traditionally has been used to describe drugs derived from opium extracts of poppy plants; “opioid” refers to compounds, such as hydrocodone, that bind to the same receptors in the human brain and body that opiates do. The mitragynine compounds work, in part, by binding to these receptors, which come in three major subtypes.
“The first one is mu — we use the Greek letter mu to signify morphine. Morphine was particularly good at this receptor subtype, and to this day the mu receptor is a predominant one,” says Solomon Snyder. A neuroscientist at Johns Hopkins University, Snyder and colleague Candace Pert characterized the mu opioid receptor for the first time in 1973, for which Snyder won the Lasker Award in 1978.
 The leaves of kratom plants in Malaysia contain at least 37 different alkaloids.Darshan singh/university of science malaysia
The leaves of kratom plants in Malaysia contain at least 37 different alkaloids.Darshan singh/university of science malaysia
The kappa receptor was named for its affinity for the drug ketocyclazocine; the delta receptor was initially discovered in the vas deferens tissue of mice. Due to a high sequence similarity with the original three opiate receptors, the receptor for the neuropeptide nociceptin often is referred to as a fourth, but it actually exhibits minimal binding activity with opioids.
“The hope was that perhaps these sites were responsible for analgesia, euphoria and, separately, for addiction, and that we could separate them out and get nonaddictive opiates,” says Snyder. “There’s been a lot of progress in having less-addicting opiates based on understanding receptor pharmacology, but to this date, there aren’t any truly nonaddicting opiates on the market.”
Heroin and morphine kill by causing respiratory depression, in which the muscles that control the diaphragm fail and breathing ceases. These overdoses can be reversed if the drug naloxone is administered within a few minutes, before irreversible brain damage sets in. Sold as Narcan, naloxone acts as an opioid receptor antagonist by essentially kicking the other opioid compounds off the opioid receptors.
According to Andrew Kruegel, a medicinal chemist and opioid researcher at Columbia University, one hypothesis in the opioid field is that fatal respiratory depression, or failure, occurs as a consequence of compounds working through the mu receptors to activate the protein beta-arrestin. When this happens at a certain threshold, a cascade of signaling that interferes with the action of the diaphragm is believed to occur.
Both mitragynine and 7-hydroxymitragynine are partial agonists for the mu receptors, binding to and activating them at less than 100 percent of the levels that other opioids, such as morphine, do. As Kruegel and colleagues observed in in vitro systems, beta-arrestin signaling is notably absent in this activity and thus may be responsible for kratom’s anecdotally reported ability to induce less respiratory depression than heroin or morphine.
The mitragynines are also both antagonists of the kappa and delta opioid receptors in in vitro systems, meaning they reverse the effects of pain-killing analgesics at these sites, but these actions aren’t yet understood in human or animal models. These receptor interactions also are hypothesized to be responsible for kratom users experiencing reduced euphoric effects and slower development of tolerance compared to those experienced with widely abused opioids.
Similar partially agonistic effects of the mu receptors are also present in buprenorphine, an opioid marketed as Buprenex and Butrans and widely used in clinics and addiction centers to help patients transition off fully agonistic opioids like heroin and morphine.
Although buprenorphine can cause a degree of respiratory depression, the compound is used in clinics because it takes an extremely high dose to induce respiratory failure. “Clinical studies have shown that (buprenorphine) has a ceiling on respiratory depression,” says Kruegel, who is examining the interactions between the components in kratom and the human opioid receptors. “You can almost think of mitragynine as buprenorphine light.”
Why kratom doesn’t appear to be as addictive as opioids, however, is not clear. Even if shutting down beta-arrestin signaling does stop respiratory depression, it doesn’t appear to be responsible for how addictive an opiatelike substance is.
“If you get rid of beta-arrestin signaling, it’s not like you’re going to have a nonaddictive opioid,” says Kruegel. “There’s a very big obsession, I would say, in the field of getting rid of the addictive properties, but I think what we should focus on is just not killing people to start with. If you get rid of respiratory depression, you’re saving 18,000 lives a year from opioid overdose, so that’s a huge improvement.”
Forgotten pharmacology
Mitragynine first was isolated from kratom leaves in 1921 by Ellen Field, a medicinal chemist at the University of Edinburgh, and its structure was first characterized by X-ray crystallography in 1964 by a group led by G.A. Jeffrey at the University of Pittsburgh.
Across the Atlantic, Joseph Shellard and Arnold Beckett at Chelsea College, London, had been examining the chemical structures of known and novel alkaloids in Mitragyna speciosa and other Mitragyna species since 1961 with Ph.D. students David Phillipson and Albert Tackie.
“The major interest was in Mitragyna speciosa, known as kratom, because it was used as a substitute for opium or as a cure for opium addiction in (what was then called) Malaya and Thailand,” says Peter Houghton, a retired pharmacognosist who also performed his Ph.D. work with Shellard.
 Kratom flowers grow in clusters at the end of the trees’ branches.American Kratom Association
Kratom flowers grow in clusters at the end of the trees’ branches.American Kratom Association
According to pharmacognosist Phillipson, now retired, there was interest in the 1960s from the pharmacy company Smith Kline French, later merged into GlaxoSmithKline, to develop mitragynine as a painkiller to replace morphine. After its potency as an analgesic was found to be more comparable to that of codeine, he says, “they thought, ‘Codeine is big business, and if this is an analgesic, then maybe it will replace codeine.’ But they did toxicity tests, and they found that mitragynine was toxic to beagle dogs, and that killed the project stone dead.”
At the time, Phillipson says, the researchers at Chelsea didn’t have enough mitragynine to undertake pharmacological research. “We obviously wanted to become involved in biological activity, and that meant collaborating with pharmacologists who would say, ‘Send me 10 grams of what you’ve got,’ but we only had 10 milligrams, and all of those have large numbers of alkaloids,” says Phillipson.
In his time with Shellard, Phillipson isolated 16 alkaloids from M. speciosa and four other Mitragyna species (for more information about kratom’s chemical components, see box “Branches bursting with secrets”).“Eight of these (alkaloids) were not previously isolated from Mitragyna, and five of them were, in fact, novel alkaloids, and I was interested in the determination of their chemical structure,” he says.
In his subsequent research, Phillipson investigated the alkaloids of Uncaria species, which are closely related to Mitragyna, as well as alkaloids of Cinchona species, the source of the antimalarial medicine quinine. All three genera belong to the same botanical family, Rubiaceae.
With interest from pharmaceutical companies gone, research into Mitragyna alkaloids as novel analgesics was halted for a number of decades.
Strange brews
Given that kratom is currently legal in the U.S., the biggest medical concern is the potential adulteration of products being sold as kratom. “The issue becomes: What degree of confidence does someone have, when someone says they’re taking kratom, that they’re actually taking kratom?” says Edward Boyer, a professor of emergency medicine at Brigham and Women’s Hospital and faculty member at Harvard Medical School.
“In the United States, there’s nothing to protect people from an adulterated product. Even truthfully, if they bought it in a store, the Dietary Supplement Health and Education Act of 1994 essentially prohibits any regulatory agency from doing anything to prevent a tainted product from reaching the marketplace,” Boyer says. “The FDA has to prove that something is unsafe, and that’s something, candidly, that the FDA never does.”
In a study published in the Journal of Medical Toxicology in 2016, Boyer and his colleagues found that a number of kratom products from a major kratom distributer based in Miami had concentrations of 7-hydroxymitragynine that were elevated notably compared with kratom leaves.
While there haven’t been any recorded deaths from overdoses resulting solely from kratom in the United States, there have been at least three deaths caused by overdosing on mixtures of kratom and other controlled substances. In addition to one death in Norway and another in Thailand, there have been nine deaths in Sweden caused by the drink “krypton,” which consists of kratom mixed with an active metabolite of the controlled painkiller tramadol.
A report from the Centers for Disease Control and Prevention published last year noted a significant increase in kratom-related calls to poison control centers between 2011 and 2015, with 49 of 660 recorded calls about kratom classified as major or life-threatening.
Additionally, legislation to outlaw the plant and its compounds was passed in Sarasota County, Florida, in 2015 after the suicide of a teenager whose toxicology report turned up positive for kratom and antidepressants. Subsequent bills were introduced in 2016 and 2017 but were strongly opposed by activists, including the American Kratom Association, and failed to pass the state legislature.
American limbo
If kratom were placed in schedule I, “the most dangerous marketers in the world would take over. They would fill the niche with black market, and they would be likely to mix it with other things,” says Jack Henningfield. Henningfield is an associate professor at the Johns Hopkins University and a vice president at the health consulting firm Pinney Associates.
When the DEA announced its intent last fall to place kratom in schedule I, which includes marijuana, LSD, psilocybin, heroin and bath salts, among other compounds, the American Kratom Association contacted Henningfield by way of the D.C.-based law firm Hogan Lovells.
 Kratom leaves are sometimes ground and sold as a powder. Emily Huff
Kratom leaves are sometimes ground and sold as a powder. Emily Huff
“We said, ‘Look, the train has left the station. The DEA will never change its opinion on this. They have yet to withdraw a scheduling proposal, as far as I know, in history,’” says Henningfield. “Nonetheless, we will help you as we can to oppose this action, because it carries serious public health risks.”
Henningfield and his colleagues previously had performed a safety analysis on kratom for another client that had been seeking to market a dietary supplement using the plant and have experience with the Controlled Substance Act’s eight-factor analysis used by the DEA and FDA to guide the regulation of potentially abusable and addictive substances, including medicines. The analyses examine eight factors of a substance relevant to scheduling: pharmacology; potential for abuse; history and current pattern of abuse; public health risk; dependence liability; scope, duration and significance of abuse; status as an immediate precursor of a controlled substance; and other current scientific knowledge.
“Like a lot of things, you could schedule it. If caffeine was a brand new drug discovered today and you brought it to the FDA, it would probably be proposed for a schedule III or IV,” says Henningfield. “It produces dependence, it’s a reinforcer, it produces pleasure, it produces physical dependence and there are withdrawal symptoms, and more.”
Instead of scheduling kratom, an ideal regulatory model would be the one used for caffeine, Henningfield says, in which a certain number of milligrams of caffeine are permitted by volume for beverages, but beans can be purchased directly to make coffee.
“Saying that it shouldn’t be scheduled isn’t saying that nothing should be done,” says Henningfield. “I think that we need information for people, labeling, oversight of the marketplace and standards that get the potential problem off the market.”
While FDA officials declined to comment for this story, the press office did say that the analysis “will be provided to DEA through (Health and Human Services) as expediently as possible.”
 Skeletal formulas of mitragynine (top) and 7-hydroxymitragynine (bottom), with the latter’s hydroxyl group in red.Wikimedia Commons
Skeletal formulas of mitragynine (top) and 7-hydroxymitragynine (bottom), with the latter’s hydroxyl group in red.Wikimedia Commons
Box of leaves
While the FDA deliberates, the pending scheduling has upended the projects of scientists who were working with kratom.
Christopher McCurdy, who was at the University of Mississippi’s National Center for Natural Products Research when the scheduling was proposed, was using mouse models to investigate kratom’s efficacy as a potential treatment for opioid withdrawal syndrome. A medicinal chemist, McCurdy has collaborated with Boyer on a number of papers, including the adulteration study.
In one experiment, McCurdy and his colleagues gave mice two doses of morphine daily for five days, doubling it each day until the mice were habituated to the substance, in order to assess kratom’s ability to prevent the symptoms of opioid withdrawal. In a normal model, the mice would then receive an opioid antagonist, such as naloxone, which is the substance used to reverse heroin overdosing, and then go through physical symptoms of opiate withdrawal. When the researchers put the mice through a five-day regimen of freeze-dried kratom tea before precipitating withdrawal, however, the animals displayed a significant reduction in side effects that was greater than that observed with methadone.
The model was subsequently repeated with extracts of pure mitragynine in place of kratom tea, which caused the residual side effects of withdrawal to vanish completely. “In the mitragynine-treated animals, we saw absolutely no withdrawal symptoms after five days,” says McCurdy.
When the ban came down, McCurdy and the pharmacologist Bonnie Avery, a collaborator who performed the analytical work on the adulteration study and prepared the dosing information for the mice models, were working on developing kratom as a botanical drug. Botanical drugs are a class created by the FDA for drugs consisting of vegetable materials with complex mixtures that can be consumed as powders, tablets, solutions and the like.
“We had to stop everything we were doing because of the pending scheduling,” says McCurdy. “We literally packed up 12 years’ worth of research and shipped it to one of our collaborators that does have a schedule I license, just in case the DEA decided to announce that it was schedule I. We would have had enough material in our possession to be considered as one with intent to distribute, so I didn’t want to be on the illegal list all of a sudden.”
McCurdy’s kratom supplies are currently in the hands of his colleague Scott Hemby, a pharmacologist at High Point University in Highpoint, North Carolina. McCurdy and Avery, who are married, recently relocated to the University of Florida in Gainesville, and are both currently faculty members in its College of Pharmacy.
“He sent me raw material — literally a box full of things,” says Hemby, who has had a schedule I license and worked with controlled substances such as heroin and cocaine for several years. Since receiving the kratom from McCurdy, Hemby has performed a number of experiments with mitragy nine and 7-hydroxymitragynine in rats.
In one self-administration experiment, Hemby and his colleagues trained rats to press a lever to receive intravenous infusions of morphine. Due to the opiate’s euphoria-inducing effects, the rats would continue pressing the lever to receive morphine. After three doses, the researchers replaced the morphine with saline, at which point the mice stopped pressing the lever. When the researchers then replaced the saline with mitragynine or 7-hydroxymitragynine, they noticed something strange.
“The animals really wouldn’t respond if you gave them mitragynine,” says Hemby. “But if we looked at 7-hydroxymitragynine, they would self-administer. So it was interesting that one of these compounds does seem to have some abuse liability and the other one doesn’t, at least in this substitution paradigm.”
A more disconcerting disparity between the two mitragynine compounds appeared when Hemby and his colleagues once again exposed rats that had gone through the same procedure to morphine.
“We gave them access to morphine again, and, interestingly, they increased their morphine intake two- to three-fold after they had a history of 7-hydroxymitragynine,” says Hemby. “Some of the newer imports are being spiked with 7-hydroxy, and that may increase subsequent morphine intake. That’s one of the subsequent concerns that we have … there are a lot of things we just simply don’t know about the mechanism behind what’s going on here.”
A scaffold for the future
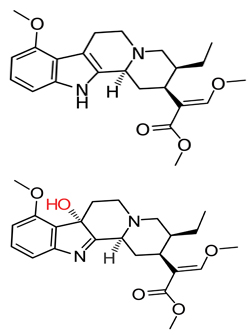
Five floors down from McCurdy’s office in the College of Pharmacy, his collaborator Jay McLaughlin is working with Susruta Majumdar at the Memorial Sloan-Kettering Cancer Center in New York to develop novel analgesics based on kratom’s molecular scaffolding.
Majumdar and McLaughlin recently published a paper in the Journal of Medicinal Chemistry detailing the analgesic effects of the modified kratom analog mitragynine pseudoindoxyl.
When Majumdar and his colleagues examined the analog’s efficacy in mouse models, “we found that it’s a potent analgesic, three to five times more potent than morphine,” says Majumdar.
 Kratom trees are grown at the University of Mississippi’s National Center for Natural Product Research. The trees can reach up to 15 meters.Christopher McCurdy/University of Florida
Kratom trees are grown at the University of Mississippi’s National Center for Natural Product Research. The trees can reach up to 15 meters.Christopher McCurdy/University of Florida
The researchers also examined its efficacy as a delta receptor-antagonist, which is a property that causes users to develop less of a tolerance to a drug that acts as a mu agonist. Across the board, pseudoindoxyl appeared to be a more potent delta antagonist in isolated in vitro receptors than either mitragynine or 7-hydroxymitragynine.
“Mitragynine pseudoindoxyl is also the first molecule in a kratom-derived template that shows no abuse potential and has far reduced respiratory depression (compared to) all clinically used opioids in mice, possibly because of its non-beta arrestin recruiting mechanisms,” he says.
Despite these benefits, “mitragynine pseudoindoxyl is more of a therapeutic for tomorrow,” he says.
In the meantime, McLaughlin is hoping to begin studies at the University of Florida with Avery and McCurdy examining pharmacodynamic properties of mitragynine pseudoindoxyl, such as its longevity in the body, byproducts and mechanisms of elimination.
Foliage frontiers
The earliest noted record of kratom use in the United States appeared in the 1999/2000 issue of the now-defunct quarterly publication Entheogen Review, a self-described “Journal of Unauthorized Research on Visionary Plants and Drugs” that ran in a confidential, underground manner from 1992 to 2008.
Today, researchers are starting to get a better idea of the demographic groups using kratom in the U.S. and their reasons for doing so. Oliver Grundmann, a toxicologist and pharmacologist at the University of Florida, recently published the initial findings from a survey of 10,000 self-reported kratom users conducted with the assistance of the American Kratom Association. The paper appeared in the journal Drug and Alcohol Dependence.
Grundmann and his colleagues found that, out of the 8,049 kratom users who completed the entire survey, less than 1 percent had experienced physical or psychological incidents that required hospitalization or treatment by a physician.
“That was a very low incidence rate, overall, compared to many other drugs … even among those that are common prescription drugs,” says Grundmann. The authors also found that, while 68 percent of kratom users were using it to self-treat pain, some 66.5 percent were using it to self-treat conditions such as anxiety, depression or post-traumatic stress disorder.
 Capsules of kratom can be purchased in varying quantities online, at herbal shops and even from vending machines in certain U.S. locations.WIKIMEDIA COMMONS USER Psychonaught
Capsules of kratom can be purchased in varying quantities online, at herbal shops and even from vending machines in certain U.S. locations.WIKIMEDIA COMMONS USER Psychonaught
“I’ve found that these effects, especially, were not necessarily as dose-dependent as some of the other effects, like the analgesic effects,” says Grundmann. “So I think that people that are taking it for the mood-elevating effects, the antidepressant effects, are not necessarily at as high of a risk of getting used to or dependent to kratom as those that are taking it for the analgesic effects or for treating opiate withdrawal symptoms.”
Kruegel and his colleagues at Columbia are seeking to demystify some of these interactions by exploring the receptor activities of kratom’s other major compounds, speciociliatine, speciogynine and panatheine, as well as elucidate mitragynine and 7-hydroxymitragynine’s interactions with the receptors to which serotonin and adrenaline molecules bind.
For the field, Grundmann says, the next stage should be human clinical trials with well-defined chemical extracts of kratom’s mitragynines and other alkaloids.
“If (clinicians) go down the route and want to use pure mitragynine and 7-hydroxymitragynine, then what we’re looking at is basically a synthetic drug derivative, like mitragynine or 7-hydroxymitragynine. Is that what we want? That’s the question: Do we want kratom to basically become a scaffold, a framework for a synthetic drug down the road, or do we want kratom extracts to remain more of a supplement that is an extract, but with a special quality standard that is established?”
At the moment, there are no concrete plans or proposals for researchers wishing to carry out clinical trials of kratom or mitragynine compounds. Grundmann is continuing to mine the survey data and hopes to have another paper published within the year.
In the meantime, medical opinion on kratom use may be shifting as regulatory agencies idle.
Among the 8,049 self-reported kratom users in Grundmann’s study, 40 percent had discussed their kratom use with their health care providers.
“I think that even within the medical community it has kind of reached a point where it’s ‘give it a try, kind of, and see if it is successful,’ instead of flat-out denying its existence or just saying it’s no good,” says Grundmann.
“The larger discussion is: How do we, as a society, respond to drugs in general, right?”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

